Abstract
A case of mistaken identity
Gilbert Berdine MD, Jose Ramos MD
Corresponding author: Gilbert Berdine
Contact Information: Gilbert.Berdine@ttuhsc.edu
DOI: 10.12746/swrccc.v11i49.1235
ABSTRACT
Images that demonstrate large and obvious abnormalities sometimes distract the provider from the careful review necessary to make the correct diagnosis. In situations in which the differential diagnosis has a leading diagnosis that is correct almost all time, it is easy to be misled by more obvious possibilities, failing to spend enough time to see the important details, and pursuing therapy that might not result in a good patient outcome. The purpose of this case report is to help readers from making analogous errors in future cases similar to this one.
Keywords: pleural effusion, endobronchial obstruction, lung cancer, complications, chest tube
INTRODUCTION
Pleural effusion is an increasing cause for pulmonary consultation. At our institution, one third to one half of pulmonary consults are due to the presence of a pleural effusion on imaging tests or are requests for procedures to drain a pleural effusion. Pleural effusions arise from a very broad range of causes, including congestive heart failure, liver cirrhosis, any cause of hypoalbuminemia and subsequent low oncotic pressure, systemic inflammatory disorders (such as lupus), infection of the lung or pleural space, malignancy, and trauma. Given that pleural effusion is a sign of some other disease rather than a specific illness itself, it is dangerous to make simplistic generalizations about pleural effusions and extrapolate these generalizations to situations in which the generalizations might not apply. For example, lung atelectasis can be caused by pleural effusion, or pleural effusion can be a consequence of lung atelectasis. Drainage of pleural effusion does not always improve the situation and, in some cases, can lead to negative outcomes. Prior to any procedural intervention for pleural effusion, the prudent practitioner should consider the etiology of the problem and the likely long-term results following an intervention.
CASE
A 76-year-old man was transferred to our facility with a left pleural effusion requiring a higher level of care. His initial symptom was cough six months prior to transfer. The patient was seen in the local hospital, diagnosed with pneumonia complicated by fluid around the left lung, treated with oral antibiotics, and sent home. The patient’s symptoms progressively worsened until about three weeks before transfer. He returned to the local hospital, was treated with a second course of antibiotics, and was sent home. The symptoms failed to improve, so the patient returned to the local hospital, where he was admitted for treatment of a large left pleural effusion. Imaging included the computed tomography (CT) image shown in Figure 1. A chest tube was placed at the local hospital. The CT image after chest tube placement is shown in Figure 2.
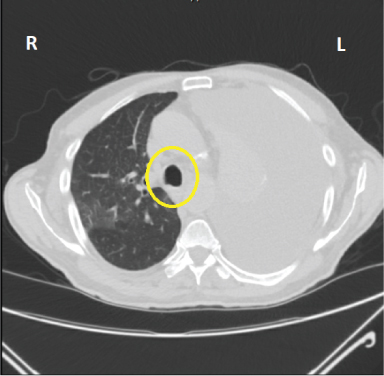
Figure 1. CT image prior to initial chest tube. Note the position of the trachea (yellow oval) to the right of midline suggesting tension from positive pleural pressure.
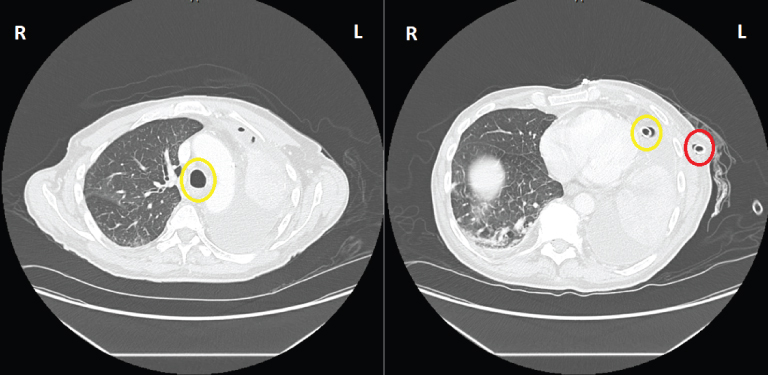
Figure 2. CT images following chest tube placement. The left panel (2a) shows the position of the trachea (yellow oval) is to the left of midline. The right panel (2b) shows the properly positioned chest tube with the tip (yellow oval) inside the thoracic cavity and the entrance (red oval) in the soft tissues outside the thoracic cavity.
Despite the chest tube being in place, the patient’s left thorax remained completely opacified, and the patient was transferred to a Level 1 hospital for a higher level of care. It is unclear what the total amount of chest tube drainage was since insertion; however, when the patient arrived at our institution, there was approximately 50cc of blood-tinged pleural fluid in the collecting system. The pulmonary consult team was asked to assist with the left pleural effusion that remained even in the presence of a surgical chest tube placed in the outside facility. Examination demonstrated absent breath sounds on the left side. A plain chest radiograph confirmed what was seen in the previous CT scan: the trachea was to the left of midline. The consult team advised against any further chest tube drainage and suggested the existing chest tube be placed to water seal to restore proper position of mediastinal structures. Despite this advice, a second larger chest tube was placed that produced approximately 150cc of pleural fluid immediately after insertion. The chest tube output decreased significantly thereafter, and no significant change in the chest x-ray was noted.
After a careful review of available imaging studies was done, a preliminary diagnosis of lung cancer with near complete occlusion of the left bronchial tree was made. As part of the work up, a diagnostic bronchoscopy was performed which revealed the absence of an endobronchial mass in the left bronchial tree, however, the lumen of the left main bronchus was severely compromised by extrinsic compression. Additionally, the appearance of the mucosa in the left main bronchus was suspicious for a submucosal tumor. To further evaluate the nature of the structure causing this compression, endobronchial ultrasound was used. This approach revealed a large extrinsic mass encasing the left main bronchus; tissue samples were obtained from fine needle aspiration via endobronchial ultrasound (Figures 3 and 4).
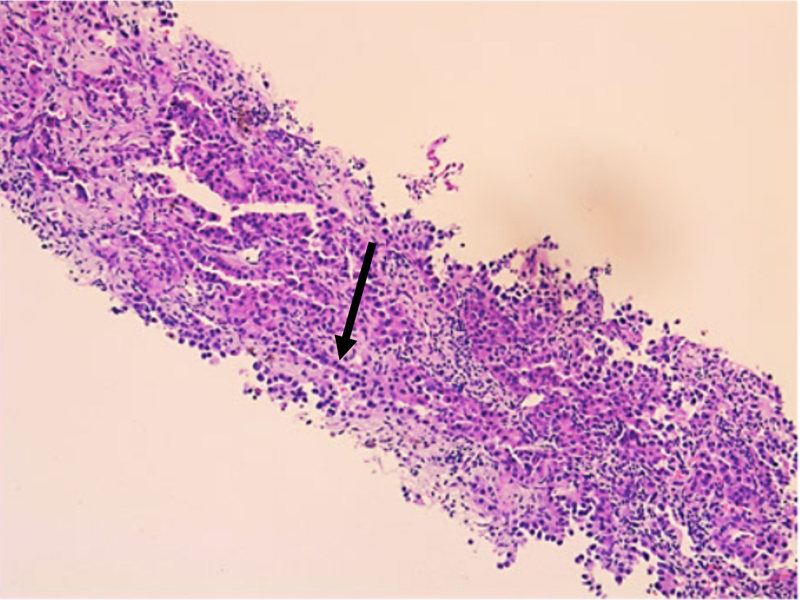
Figure 3. Left lung mass biopsy with H&E stain at 10x magnification showing tumor cells with hyperchromatic nuclei (arrow).

Figure 4. Left lung mass biopsy with H&E stain at 40x magnification depicting tumor cells with hyperchromatic nuclei (arrow).
DISCUSSION
In this case, the identity of the left thorax opacification or left hemithorax was thought to be solely secondary to a large pleural effusion; however, careful review of the imaging revealed that the true identity was complete atelectasis of the left lung due to obstruction of the left bronchial tree.
An important complication to keep in mind when dealing with this type of scenario is the development of a trapped lung, which was seen in this case. The term “trapped lung” refers to the inability of the lung to re-expand and fill the thoracic cavity.1 This is caused by conditions in which the lung parenchyma is surrounded by a fibrous rind or pleural peel trapping the lung and preventing re-expansion when pleural fluid is removed. Typically, active inflammation of the pleura due to infection or malignancy is the cause of this fibrinous layer formation.2 Pleural manometry during thoracentesis is a useful method to identify a trapped lung when the suspicion is high, as supported by the presence of certain risk factors, including the presence of a chronic pleural effusion.2
Atelectasis is a partial or complete collapse of the lung and can occur due to three mechanisms: airway obstruction by endobronchial mass, foreign body, or mucus plugs; external compression of the airway from extrathoracic, intrathoracic or chest wall processes; and increased surface tension in the alveoli.3 When atelectasis results in a completely opacified-hemithorax, serious diagnoses, such as a tumor, must be considered. In this case, the atelectasis of the left lung was caused by an airway obstruction of the left main bronchus secondary to the external compression of the left main bronchus by the large parenchymal adenocarcinoma. The key finding to make the correct diagnosis was the review of radiological images showing that the position of the trachea had shifted to the side of the opacity (Figure 2). In cases of complete atelectasis, the mediastinal structures shift to the ipsilateral side. When reviewing images in the setting of an opacified hemithorax, it is important to identify any shifts in mediastinal structures, hemidiaphragm, and upper abdominal structures which if present, would indicate volume loss.4
Tracheobronchial obstructions can result in persistent cough, dyspnea, wheezing and ventilatory impairment.5 The treatment can vary depending on the etiology of atelectasis. For obstructive atelectasis, fiberoptic bronchoscopy is important in management and should always be considered in these cases as this intervention can identify the cause and relieve the atelectasis in 76% of the cases.6
REFERENCES
- Doelken P, Sahn SA. Trapped lung. Semin Respir Crit Care Med. 2001 Dec;22(6):631–6. doi: 10.1055/s-2001-18799.
- Huggins JT, Doelken P, Sahn SA. The unexpandable lung. F1000 Med Rep. 2010 Oct 21;2:77. doi: 10.3410/M2-77.
- Peroni DG, Boner AL. Atelectasis: mechanisms, diagnosis and management. Paediatric Respiratory Reviews 2000;1(3): 274–278. doi.org/10.1053/prrv.2000.0059.
- Rosado de Christensen, Melissa, and others (eds), Chest Imaging, Rotations in Radiology (New York, 2019; online edn, Oxford Academic, 1 July 2019), https://doi.org/10.1093/med/9780199858064.001.0001
- Chen H, Hong X, He Y. Successful treatment of bronchial obstruction by flexible bronchoscopy and isoniazid: A case report. Exp Ther Med. 2014 Feb;7(2):397–400. doi: 10.3892/etm.2013.1423.
- Grott K, Chauhan S, Dunlap JD. Atelectasis. [Updated 2022 Oct 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK545316/
Article citation: Berdine G, Ramos J. A case of mistaken identity. The Southwest Respiratory and Critical Care Chronicles 2023;11(49):31–34
From: Department of Internal Medicine, Texas Tech University Health Sciences Center, Lubbock, Texas
Submitted: 3/17/2023
Accepted: 9/16/2023
Conflicts of interest: none
This work is licensed under a Creative Commons
Attribution-ShareAlike 4.0 International License.