Abstract
Teprotumumab-associated hearing-related adverse events
Amanda J. Key BS, Addie M. Pederson BA, Jared P. Sant BS, Coby N. Ray MD, MS
Corresponding author: Coby Ray
Contact Information: Coby.Ray@ttuhsc.edu
DOI: 10.12746/swrccc.v12i50.1261
ABSTRACT
This study aims to provide a review of the existing literature on teprotumumab (Tepezza)-associated hearing-related adverse effects. A review of PubMed and Embase was conducted using keywords “teprotumumab,” “tepezza,” “hearing disorder[s],” “hearing loss,” and “ototoxicity.” These search results were filtered to include all clinical trials, observational studies, case reports, and case series relevant to the topic of teprotumumab-associated hearing disorders. Data collection from the 15 included studies consisted of: sample size, number and percentage of hearing disorders reported, types of hearing disorders, remission rates, timeline of symptom onset, predisposing risk factors, suggested screening guidelines, and treatment proposals. Teprotumumab-associated hearing disorders are reported in 7–81.5% (median 12%) of clinical study participants. Symptoms described include sensorineural hearing loss (SNHL), hypoacusis, autophony, ear fullness/pressure/plugging, patulous eustachian tube, and tinnitus. Most symptoms improve with discontinuation of teprotumumab, but some symptoms persist after completion of treatment, most commonly SNHL. Symptoms have been reported occurring 3–37 (median 8.4) weeks after treatment initiation, with the majority reported 6 weeks after treatment initiation. Additional prospective studies are needed to clarify how frequently teprotumumab causes ototoxicity. There remains a need for both standardized audiologic screening guidelines and treatment for patients in whom ototoxicity persists post-treatment.
Keywords: teprotumumab, tepezza, hearing disorder[s], hearing loss, ototoxicity
INTRODUCTION
Thyroid eye disease (TED) is a rare autoimmune condition associated with Graves’ disease, an autoimmune disorder that leads to the overproduction of thyroid hormone.1 Some of the more common symptoms associated with TED are proptosis, diplopia, edema of the eyelids, varying degrees of orbital inflammation, and pain around the eye that worsens with eye movement.1 The exact pathophysiology of TED is still under investigation, but it is thought to be closely associated with activation and upregulation of thyroid stimulating hormone receptors (TSHRs) and overexpression of insulin-like growth factor 1 receptors (IGF-1Rs) in orbital fibroblasts through cross-talk between the two receptors.2 The activation of these overexpressed receptors causes an increase in the amount of T-cell chemoattractants which leads to the inflammation and tissue remodeling that is characteristic of TED.2
In 2020, the United States Food and Drug Administration (FDA) approved the monoclonal antibody teprotumumab (Tepezza) for the treatment of TED. At this time, it is the only FDA-approved treatment for TED on the market. Teprotumumab works by inhibiting the IGF-1R and reducing the downstream effects of IGF-1 which likely has a role in the pathogenesis of TED. This monoclonal antibody is administered over a six-month period, with infusions given every 3 weeks for a total of 8 infusions. Phase II and phase III randomized controlled clinical trials (RCTs) have proven clinical efficacy for use of teprotumumab in treatment of TED with a relatively minimal side effect profile.3,4 One of the more significant side effects reported, however, is hearing dysfunction. Since the RCTs have been published, multiple other clinical studies and case reports have described similar ototoxicity. The pathogenesis underlying this adverse effect is hypothesized to be due to the role of IGF-1 in the protection and survival of cochlear hair cells and in the autophagy of otic neural processors.5,6 Inhibition of the IGF-1R has been closely associated with a decline in auditory sensitivity.7
Although the frequency of this adverse event varies in clinical and observational studies, it is undoubtedly high enough that ophthalmologists, audiologists, and otolaryngologists must be aware of the ototoxic potential of teprotumumab. At the time of publication, there is no review available that summarizes all current scientific literature regarding teprotumumab’s effects on hearing. This narrative literature review will attempt to correct this deficiency by analyzing the information from 6 clinical studies3,4,8–11 and 9 case reports/case series.12–20
METHODS
A review of the existing literature on teprotumumab-associated ototoxicity was conducted. This research is HIPAA-compliant and adhered to the ethical principles outlined in the Declaration of Helsinki as amended in 2013. Databases PubMed (Medline) and Embase were searched and all literature from 2017 to March 2023 was included. PubMed was searched using the query “(teprotumumab OR tepezza) AND (hearing disorders OR hearing loss OR ototoxicity)” then filtered through the “English & Humans” NCBI filter, returning 17 hits. Embase was searched using the query “(‘teprotumumab’/exp OR ‘teprotumumab’ OR ‘tepezza’/exp OR ‘tepezza’) AND (‘hearing disorder’/exp OR ‘hearing disorder’ OR ‘hearing loss’/exp OR ‘hearing loss’ OR ‘ototoxicity’/exp OR ‘ototoxicity’)” and filtered through “human” study type, returning 54 hits. Opinion or comment pieces, conference abstracts, and reviews of the phase II and III RCTs were excluded from further data collection. The phase II and phase III RCTs referenced by the reviews, however, were included.3,4 Several other publications were excluded from further review based on irrelevance to our study topic.21–23 This search method resulted in 3 clinical trials, 2 observational studies, 1 cohort, 6 individual case reports, and 3 case series included in this literature review for further data collection.
Three independent reviewers performed data collection. Data extracted from the articles included: sample size (n), number and percentage of reported hearing disorders, types of hearing disorders reported, rates of remission, when along the treatment course symptoms were reported, any predisposing risk factors, and proposed screening and treatment for teprotumumab-associated ototoxicity. The data from the clinical studies for number and percentage of reported hearing disorders and types of hearing disorders were compiled into a table (Supplemental Table 1). Information regarding the chronology of symptom occurrence was synthesized into a timeline graphic (Figure 1). All remaining extracted data were analyzed in the discussion to present the current information on this topic and future directions for advancement in this field.
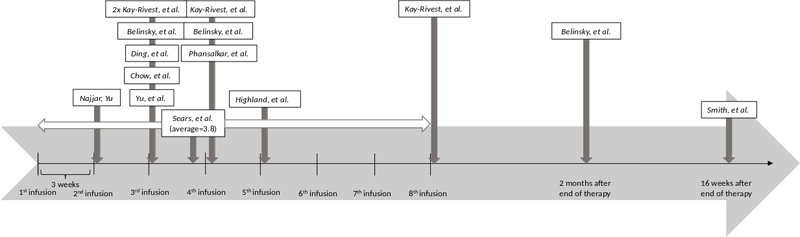
Figure 1. Timeline of onset of hearing disorder symptoms for studies including this information.3,8,11–13,15,16,18–20 Hatch marks represent teprotumumab infusions, occurring every 3 weeks for a total of 8 infusions. Najjar and Yu report a case occurring after the second infusion. Yu et al., Chow et al., and Din, et al., report cases occurring after the 3rd infusion. Belinsky et al. report one case occurring after the 3rd infusion, one occurring after the 4th infusion, and one occurring 2 months after completion of therapy. Kay-Rivest et al. report two cases occurring after the 3rd infusion, one occurring after the 4th infusion, and one occurring after completion of treatment. Sears et al. report new or worsening symptoms occurring on average after 3.8 infusions, ranging from after the 1st infusion to after the 8th infusion. Phansalkar et al. report a case occurring after the 4th infusion. Highland, et al. reports a case occurring after the 5th infusion. Smith et al. report a case occurring 16 weeks after completion of therapy.
RESULTS
The prevalence of hearing disorders associated with teprotumumab use varies from 7–81.5% (median 12%) (Supplemental Table 1). The RCTs suggest a prevalence of 7–12.2%,3,4,9 but more recent prospective observational studies suggest the prevalence is higher than originally thought.8,11 Hearing disorders described by the studies include new or worsening sensorineural hearing loss (SNHL), hypoacusis, autophony (abnormal sound of one’s own voice), ear fullness/pressure/plugging, patulous eustachian tube (PET), and tinnitus (Table 1). Of the types of hearing disorders reported, SNHL/hypoacusis were the most common symptoms. Symptom onset ranged from after the first infusion to 16 weeks after the completion of treatment, or 27 weeks after treatment initiation (Figure 1). The most common timing for symptom onset is between the third and fourth infusions, or 6–9 weeks after treatment initiation. Rates of remission of teprotumumab-associated hearing disorders vary from 33–100% (median 50%) upon cessation of treatment.3,4,8–10 Sears et al. note the symptoms most likely to resolve are tinnitus (100%) and ear fullness/pressure/plugging (90.9%) while the symptom least likely to resolve is SNHL (45.5%).
Table 1. Reported Hearing Disorder Subtypes
| Study |
n |
# Hearing Disorders (%) |
Hearing Disorder Subtype |
| Hearing Loss/Hypoacusis |
Autophony |
Ear Fullness/Pressure/Plugging |
Patulous Eustachian Tube |
Tinnitus |
| Smith, et al. |
43 |
3 (7%) |
2 |
|
|
|
1 |
| Douglas, et al. (OPTIC) |
41 |
5 (12%) |
3 |
1 |
|
1 |
|
| Douglas, et al. (OPTIC-X) |
51 |
6 (12%) |
3 |
1 |
|
|
2 |
| Sears, et al.* |
27 |
22 (82%) |
11 |
7 |
13 |
1 |
10 |
| Ho, et al.† |
74 |
8 (11%) |
|
|
|
|
|
| Kay-Rivest, et al.* |
35 |
15 (43%) |
15 |
1 |
6 |
3 |
5 |
DISCUSSION
There is a wide variance in the prevalence of reported hearing disorders between the clinical and observational studies. This difference may be in part attributed to the use of both objective and subjective measures for data collection in observational studies, the two of which do not always correlate. It is possible the prevalence was higher in the RCTs but not perceived and reported by study participants due to the lack of objective testing in place at the time of the trial. Because of this substantial variance, additional prospective studies collecting both objective and subjective data are needed to more accurately determine the risk of ototoxicity with use of teprotumumab.
Several risk factors contributing to ototoxicity have been proposed, including baseline SNHL, the use of teprotumumab concomitantly with other ototoxic agents, and loud noise exposure.8,12,14,15 Sears et al. found a statistically significant incidence of hearing-related adverse events in patients who had prior audiologic conditions. Notably, they also found an increase in hearing-related adverse events in older patients (average of 70.8 years vs. 55.4 years); however, this was not statistically significant. Chow et al. reported a case of chronic teprotumumab-associated SNHL in a patient with mild hearing loss demonstrated at baseline audiometry. Highland et al. suggested caution in using teprotumumab with another ototoxic agent or with noise exposure exceeding 70 dB. Reed et al. described a case of a patient on teprotumumab with sustained hearing loss secondary to a rifle blast, suspecting the teprotumumab increased the susceptibility of the cochlear hair cells to noise-induced trauma. All patients beginning treatment with teprotumumab should be counseled on its ototoxic effects, but extra caution should be taken with patients exhibiting these predisposing factors. Patients with SNHL at baseline audiometry testing, currently using other agents known to cause ototoxic effects, with occupational or recreational exposure to loud noises, or older age should be forewarned that they may be more likely to experience the ototoxic effects of teprotumumab.
There remains a need for regular audiologic screening guidelines for patients undergoing treatment of TED with teprotumumab. Four of the included articles suggest broad guidelines for consideration.8,15,16,18 Sears et al. suggest audiologic screening in all patients, especially patients exhibiting baseline hearing loss due to the identification of these patients at higher risk for developing more hearing loss during treatment. Highland et al. recommend obtaining a pre-treatment audiogram for all patients prior to initiating teprotumumab therapy, implementing the standard of practice set in place by other known ototoxic drugs, such as cisplatin. Yu et al., recommend screening all patients using teprotumumab before and after treatment to detect objective changes in hearing function. Finally, Belinsky et al. suggest one screening prior to initiating treatment, one screening in the middle of treatment, and one screening 6 months after completion of treatment until the results of more prospective investigations are available and a standardized guideline exists.
The absence of such standardized screening guidelines highlights the need for close audiologic monitoring during treatment and follow-up for all patients. Frequent screening will allow teprotumumab administration to be promptly discontinued upon complaints of worsening or new-onset hearing disorders, especially SNHL or hypoacusis since these are the symptoms least likely to spontaneously resolve. Ophthalmologists will likely need to involve audiologists or otolaryngologists in collaborative care of these patients. Baseline audiologic testing including SNHL and PET testing is useful to determine objective changes in hearing attributed to teprotumumab. Subjective symptoms should also be monitored to recognize symptoms such as tinnitus, autophony, and ear fullness/pressure/plugging. Development of a standardized questionnaire to assess these subjective symptoms consistently is still needed. This testing should be repeated during treatment and after conclusion of treatment, though the frequency of testing remains yet to be determined. We agree with the utility of a mid-treatment test proposed by Belinsky et al. due to the high incidence of hearing disorders reported after the third infusion. Furthermore, the description of a case of hearing loss reported 16 weeks after conclusion of treatment emphasizes the importance of long-term audiologic follow-up for these patients.3
Although most hearing-related adverse events resolve with cessation of teprotumumab administration, there remains a need for standardized treatment for patients with persisting hearing dysfunction. Various suggestions have been proposed by 4 case reports.12,13,16,19 Chow et al. suggest that local IGF-1 injections might aid in teprotumumab-associated hearing loss since the pathogenesis of the hearing loss involves localized depletion of IGF-1. This treatment modality currently consists of an intratympanic injection, but they propose a novel treatment of chemical permeation-enhanced hydrogel IGF-1 formulation as a potential horizon for external delivery such as ear drops. Ding et al. discusses the various treatment options currently used for other ototoxic drugs, such as aminoglycosides and cisplatin. They suggest that newer treatments that are effective for other drug-induced ototoxicity, like sodium thiosulfate, N-acetylcysteine, and amifostine, might be effective for teprotumumab-associated hearing loss. These suggestions for potential treatments remain yet to be validated in clinical practice. Belinsky et al. state that the best treatment for non-self-limited hearing loss due to teprotumumab is the use of hearing aids. Finally, Phansalkar et al. discussed a successful reduction in hearing related adverse events by transitioning to half- dose teprotumumab therapy. In this case study, a patient who had experienced objective and subjective hearing loss, autophony, and ear popping on full dose teprotumumab discontinued her treatment after 4 infusions. One year later, she restarted half dose teprotumumab therapy and completed all 8 infusions with no worsening of objective hearing loss or autophony. This in combination with the Ho et al. study demonstrating clinical improvement of TED even with partial treatment suggests dose reduction, either through a lower quantity given per administration or fewer total treatments, is a promising prospect for achieving therapeutic response to teprotumumab while minimizing adverse events.
Limitations to this narrative literature review include a finite amount of existing literature on this topic. This review consists of 15 publications, only 6 of which are clinical trials. Future studies and control trials will reduce the variance of the data and guide clinical decision making.
REFERENCES
- Al-Zubidi, MD N. Thyroid eye disease. EyeWiki. Available at: https://eyewiki.org/Thyroid_Eye_Disease#History.2FSymptoms. Published July 10, 2022. Accessed February 23, 2023.
- Pritchard J, Han R, Horst N, et al. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves’ disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol 2003;170:6348–54.
- Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med 2017 May 4;376(18):1748–1761.
- Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med 2020 Jan 23;382(4):341–352.
- Yamahara K, Nakagawa T, Ito J, et al. Netrin 1 mediates protective effects exerted by insulin-like growth factor 1 on cochlear hair cells. Neu ropharmacology 2017 Jun;119:26–39.
- Aburto MR, Sánchez-Calderón H, Hurlé JM, et al. Early otic development depends on autophagy for apoptotic cell clearance and neural differentiation. Cell Death Dis 2012 Oct 4;3(10):e394.
- Rodríguez-de la Rosa L, Lassaletta L, Calvino M, et al The Role of Insulin-Like Growth Factor 1 in the Progression of Age-Related Hearing Loss. Front Aging Neurosci 2017 Dec 12;9:411.
- Sears CM, Azad AD, Amarikwa L, et al. Hearing dysfunction after treatment with teprotumumab for thyroid eye disease. Am J Ophthalmol 2022 Aug;240:1–13.
- Douglas RS, Kahaly GJ, Ugradar S, et al. Teprotumumab efficacy, safety, and durability in longer-duration thyroid eye disease and re-treatment: OPTIC-X Study. Ophthalmology 2022 Apr;129(4):438–449.
- Ho TC, Maamari RN, Kossler AL, et al. Outcomes of patients with thyroid eye disease partially treated with teprotumumab. Ophthalmic Plast Reconstr Surg. 2023 Mar–Apr 01;39(2):150–155.
- Kay-Rivest E, Belinsky I, Kozlova A, et al. Prospective assessment of otologic adverse events due to teprotumumab: preliminary results. Otolaryngol Head Neck Surg. 2023 May;168(5):1164–1169.
- Chow A, Silkiss RZ. Teprotumumab-associated chronic hearing loss screening and proposed treatments. BMJ Case Rep 2022 Apr 13;15(4):e248335.
- Ding AS, Mahoney NR, Campbell AA, et al. Sensorineural hearing loss after teprotumumab therapy for thyroid eye disease: A Case Report. Otol Neurotol 2022 Feb 1;43(2): e148–e152.
- Reed DS, Kostosky N, Davies BW, et al. Rifle blast exacerbating hearing loss in a patient treated with teprotumumab for thyroid eye disease. Ophthalmic Plast Reconstr Surg 2022 Mar–Apr 01;38(2):e41–e43.
- Highland J, Gordon S, Reddy D, et al. Ototoxicity and Teprotumumab. Ann Otol Rhinol Laryngol 2022 Aug;131(8):910–913.
- Belinsky I, Creighton FX Jr, Mahoney N, et al. Teprotumumab and hearing loss: case series and proposal for audiologic monitoring. Ophthalmic Plast Reconstr Surg 2022 Jan–Feb 01;38(1):73–78.
- Vinson KB, Kirzhner M. Effects of teprotumumab on patients with long-standing, active thyroid eye disease. Am J Ophthalmol Case Rep 2022 Jan 26;26:101348.
- Yu CY, Correa T, Simmons BA, et al. Audiology findings in patients with teprotumumab associated otologic symptoms. Am J Ophthalmol Case Rep 2021 Sep 16;24:101202.
- Phansalkar R, Lu T, Alyono J, et al. Reduction of teprotumumab-induced hearing loss with comparable efficacy using half-dose therapy. Ophthalmic Plast Reconstr Surg. 2023 Jul–Aug 01;39(4):e101–e104.
- Najjar W, Yu J. Audiologic demonstration of ototoxicity from teprotumumab treatment in a patient with thyroid eye disease. OTO Open. 2022 Apr 29;6(2):2473974X221097097.
- Mervis JS, Maeng MM, Kirsner RS, et al. Teprotumumab as a novel treatment for pretibial myxoedema. Br J Dermatol 2022 Nov;187(5):795–797.
- Houen G. Therapeutic antibodies: An overview. Methods Mol Biol 2022;2313:1–25.
- Safo MB, Silkiss RZ. A case of ulcerative colitis associated with teprotumumab treatment for thyroid eye disease. Am J Ophthalmol Case Rep 2021 Mar 10;22:101069.
Article citation: Key AJ, Pederson AM, Sant JP, Ray CN. Teprotumumab-associated hearing-related adverse events. The Southwest Respiratory and Critical Care Chronicles 2024;12(50):24–29
From: Texas Tech University Health Sciences Center School of Medicine (AJK, AMP); Department of Ophthalmology (CNR), Texas Tech University Health Sciences Center, Lubbock, Texas; University of Arizona (JPS), Tucson, Arizona
Submitted: 11/28/2023
Accepted: 1/17/2024
Conflicts of interest: none
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.