INTRODUCTION
Adipose tissue has many functions, especially in energy homeostasis and lipid metabolism. It also modulates other physiologic processes, including innate and adaptive immune responses, insulin sensitivity, and body temperature regulation. During periods of critical illness, adipose tissue can undergo significant changes in morphology, gene expression, and cytokine secretion.1–3 These changes affect energy availability, steroid hormonogenesis, and immune system activity—all functions considered particularly significant in the intensive care patient. As the understanding of adipose tissue and its effects on normal and disease-state physiology continues to increase, the composition, distribution, and activity of adipose tissue in patients may interest critical care specialists.
Computed tomography (CT) is considered the preferred method for measuring adipose tissue mass in critically ill patients.4,5 However, it has several disadvantages, namely its use of ionizing radiation and the inconvenience associated with transporting patients outside of the intensive care unit (ICU) to obtain scans. The convenience of performing scans at the bedside without exposing patients to ionizing radiation makes ultrasonography an attractive option for monitoring fat composition in critically ill patients. Currently there is no widely accepted protocol to quantify subcutaneous or visceral adipose tissue using point-of-care ultrasonography. This paper reviews the relevance of adipose tissue in critical illness and ultrasound techniques used to assess body fat composition in the ICU.
ADIPOSE TISSUE—COMPOSITION, TYPES, AND DISTRIBUTIONS
Adipose tissue is a loose connective tissue found throughout the body. It is composed of adipocytes, which make up 90% of adipose tissue by volume, and several other supporting cells, including mesenchymal stem cells, preadipocytes, fibroblasts, macrophages, T lymphocytes, and vascular endothelial cells. There are two main types of adipose tissue, white adipose tissue and brown adipose tissue. White adipose tissue is the most abundant type found in adults and is localized to the hypodermis (subcutaneous adipose tissue, SAT), intrathoracic and intra-abdominopelvic cavities (visceral adipose tissue, VAT), muscle compartments, bone marrow, and other distinct depots (Table 1). Brown adipose tissue is found abundantly in newborns and hibernating mammals but is absent or undetectable in most adult men and women.
While once considered only inert storage sites for lipids and lipid-soluble molecules, adipose tissue is now known to be an integral part of the human endocrinologic system. Adipocytes secrete hundreds of unique bioactive peptides (adipocyte-specific adipokines) capable of regulating mood, appetite, insulin sensitivity and secretion, blood pressure, hemostasis, wound healing, and humoral and cellular immunity. Macrophages and other cells within the stromal vascular fraction of adipose tissue secrete additional cytokines, including TNF-α, IL-1β, and IL-6, which modulate adipogenesis and immune system activity. Therefore, adipose tissue has been described as another endocrine organ.7 Alterations in adipose tissue function inevitably lead to changes in other organ systems.
RELEVANCE OF ADIPOSE TISSUE IN THE INTENSIVE CARE SETTING
Patients with excess adipose tissue (i.e., obesity) have different respiratory, cardiovascular, and metabolic characteristics than patients with normal amounts of adipose tissue.8–10 For example, patients with obesity expend more energy to breathe, have lower respiratory compliances, and may benefit from different mechanical ventilation strategies (e.g., higher positive end-expiratory pressures in the setting of acute respiratory distress syndrome) than patients without obesity.8,11–12 Observational studies have demonstrated that people with more visceral adipose tissue have higher serum levels of acute phase reactants, including C-reactive protein, IL-6, and TNF-α.13,14 Consequently, some authors have described obesity as a condition of “chronic, low-grade inflammation.”15
Obesity confers an increased risk of all-cause mortality in the general population.16 In the critically ill, however, obesity has been repeatedly shown to confer a paradoxical survival advantage. Recent meta-analyses have demonstrated that body mass indices (BMIs) between 25 kg/m2 and 40 kg/m2 are associated with decreased mortality from sepsis, acute respiratory distress syndrome (ARDS), and critical illness in general than BMIs less than 25 kg/m2 or more than 40 kg/m2.17–19 A retrospective study of 160,940 ICU patients found the BMI associated with the lowest likelihood of all-cause mortality in the ICU is 28.3 (Figure 1).20 This survival benefit exists even though patients with BMIs above 25 are more likely to develop acute kidney injury, ARDS, and catheter-related infections in the ICU, and may be less likely to be recognized as malnourished than patients with normal or underweight BMIs.21–25
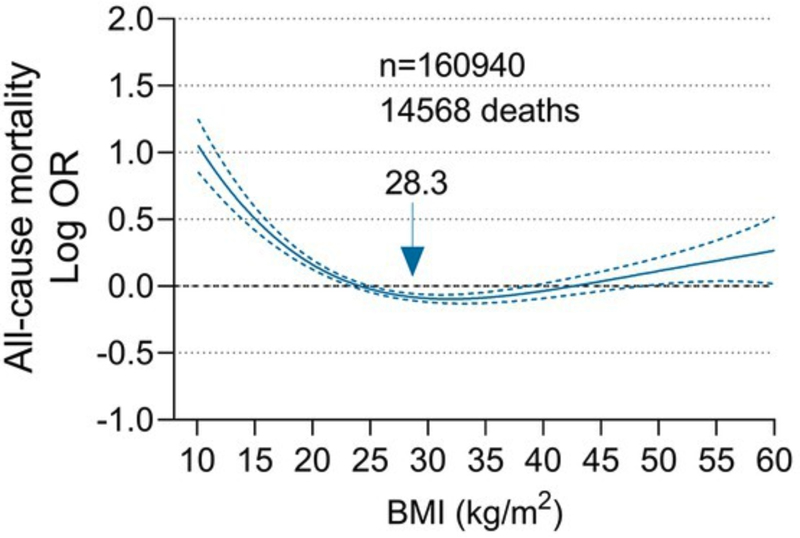
Figure 1. Association between BMI and all-cause mortality based on a sample of 160,940 patients in the eICU Clinical Research Database. Figure reproduced from Li et al. (2023) under Creative Commons 4.0 License.
Some authors have pointed out that when adjusted for confounding variables, such as nutritional status, the paradoxical survival advantage of higher BMIs disappears.26 Others have shown that when adipose tissue mass is directly measured, excessive adipose tissue in certain compartments (visceral, intermuscular, and epicardial adipose tissue) is actually associated with worse survival in critically ill patients.27–29 The true relationship between body composition and outcomes in the critically ill remains uncertain. However, these studies suggest that quantifying adipose tissue directly with imaging techniques may be useful in predicting outcomes or assessing nutritional status in the ICU.
CURRENT TECHNIQUES FOR QUANTIFYING ADIPOSE TISSUE IN THE CRITICALLY ILL
Anthropometric techniques (e.g., height, weight, BMI, body circumferences, skinfold thickness) are the most commonly used methods to evaluate body composition in the critically ill. More advanced techniques, including bioelectrical impedance analysis, dual-energy X-ray absorptiometry (DXA), air displacement plethysmography (ADP), magnetic resonance imaging (MRI), CT, and ultrasound, have been used in pediatric and adult intensive care units (ICUs).30 The ideal technique for quantifying adipose tissue in critical care settings should be safe and cost-effective for the patient, as well as accurate and reproducible. With 15–20% of ICU patients being obese, it is important to consider accessibility when choosing an imaging modality.
Anthropometric measurements, such as BMI, waist circumference, and waist-to-hip ratio offer convenient and accessible ways to categorize adipose tissue. However, these methods are unable to directly quantify adipose tissue mass or differentiate between visceral and subcutaneous adipose tissue. Although bioelectrical impedance analysis can be used to estimate total fat mass, it cannot differentiate between visceral and subcutaneous fat. It has also not been validated in critically ill populations and is unreliable in people with non-standard distributions of water and electrolytes, such as patients with chronic kidney disease and patients undergoing rapid changes in hydration status.31,32
Air displacement plethysmography and DXA provide indirect measurements of visceral adipose tissue volume. Air displacement plethysmography uses pressure-volume relationships to predict body volume and body density. While DXA is more commonly used to measure bone density, it also has the capacity to assess fat composition. Both air displacement plethysmography and DXA are promising methods for analyzing body composition; however, due to their inability to distinguish between different types of adipose tissue, they may have less utility than CT or ultrasonography. As ADP and DXA use becomes more common in clinical settings for measuring body composition, more research is needed to develop methods for accurate readings.
Computed tomography is currently considered the gold standard method for quantifying adipose tissue volumes in healthy and ill patients.33,34 It can differentiate between all types of white adipose tissue and has proven accuracy in differentiation of visceral and subcutaneous adipose tissue; however, the expense, exposure to radiation, and inconvenience of the inability to perform the scan bedside are factors that make it a less useful as an imaging modality for monitoring fat composition in the critical care setting.
Ultrasound imaging can be performed at the bedside, does not expose patients to ionizing radiation, and can be used to quantify visceral, subcutaneous, and epicardial fat. At the moment, there is no widely accepted protocol to quantify adipose tissue using point-of-care ultrasonography. In the following section, we review the various ultrasound techniques currently used to assess body fat composition.
QUANTIFYING ADIPOSE TISSUE WITH ULTRASONOGRAPHY—A REVIEW OF TECHNIQUES
In 1965, Bullen et al. used ultrasonography to measure SAT thickness in 100 subjects—the first reported study of its kind.35 They used a probe with an early, one-dimensional scanning mode called “A-mode” (“A” standing for “Amplitude”) to record measurements at three sites: on the triceps, below the scapula, and two centimeters below and to the right of the umbilicus. The measurements were compared to those made by skinfold thickness and core needle biopsies. The results demonstrated an excellent correlation between ultrasonographic and needle puncture measurements, especially at the abdominal site (r = 0.98).
Many methods have since been proposed to measure SAT thickness. The most accurate technique is the standardized protocol established by the International Olympic Committee Medical Commission.36 This protocol measures SAT thickness at eight sites: upper abdomen, lower abdomen, erector spinae, distal triceps, brachioradialis, lateral thigh, front thigh, and medial calf (Figure 2). All sites have clearly visible fascial layers, no nearby complex structures, and little variation in SAT thickness around the site. Due to the high resolution of ultrasound, this protocol is considered more accurate and precise than other imaging methods, including MRI and CT.37 However, due to the number of sites that require imaging, repeat examinations may be cumbersome. A technique more suitable for repeat measurements of SAT thickness might be the one proposed by Suzuki et al., which involves scanning the xipho-umbilical line to determine the point of maximum SAT thickness (Figure 3).38 This examination is accurate, repeatable, reproducible, and quick to perform. For these reasons it is one of the more common techniques used in clinical practice today.39
Epicardial adipose tissue is a topic of increasing interest in metabolic endocrinology. After an autopsy study of 200 hearts in 1989 found significant correlation between epicardial adipose tissue thickness and SAT thickness, researchers began investigating epicardial adipose tissue as a predictor of metabolic disease.44 The first report quantifying epicardial adiposity in vivo was provided by Iacobellis et al. in 2003.45 In this study, the authors performed transthoracic echocardiography on healthy patients lying in the left lateral decubitus position. While imaging the right ventricle in both parasternal short- and long-axis views, the authors measured the epicardial adipose tissue thickness originating on the free wall of the right ventricle. These measurements correlated well with epicardial fat measurements taken via cardiac MRI (r = 0.91, p = .001) and with VAT estimated by abdominal MRI (r = 0.84, p = .01). Further studies have validated the correlation between epicardial adipose tissue and VAT.46 In critically ill patients, epicardial adipose tissue volume has been associated with early mortality in certain diseases, like COVID-19, but its relationship to survival in other critical illnesses is unclear.47
WHICH ULTRASONOGRAPHIC TECHNIQUE SHOULD BE USED IN CRITICALLY ILL PATIENTS?
The most accurate technique to measure SAT is the standardized, eight-site protocol used by the International Olympic Committee Medical Commission. In theory, it is the best method to assess baseline SAT volume and changes in SAT volume that occur throughout an ICU stay. However, this technique does not assess VAT, the adipose tissue depot that is considered most deleterious to health and may be too cumbersome to repeat frequently in the ICU due to its number of sites and the location of sites on both anterior and posterior surfaces that would require moving the patient to acquire. The Iacobellis (transthoracic) approach is the method of choice for imaging epicardial adipose tissue. However, while epicardial adipose tissue is an interesting adipose tissue depot that may be important in cardiovascular and metabolic health, its importance in critically ill patients is not well characterized, and currently there is little clinical utility in using this approach.
The Armellini (supraumbilical) and Suzuki (xipho-umbilical line) approaches are the most suitable techniques for adipose tissue quantification in the ICU setting due to their ease of use, reproducibility, and relative accuracy. The Armellini method requires only one site of examination, whereas the Suzuki method may be more accurate and less likely to underestimate adiposity. While the Armellini approach was initially designed to measure VAT, and the Suzuki approach was initially designed to measure SAT, both techniques can be used to measure either adipose tissue depot.
CONCLUSION
Bedside ultrasonography offers a convenient, radiation-free way to quantify adipose tissue volumes in both healthy and ill patients. The Armellini (supraumbilical) and Suzuki (xipho-umbilical line) approaches appear to be the most suitable techniques for quantifying VAT and SAT in the intensive care setting due to their ease of use, reproducibility, and relative accuracy. Establishing standardized protocols to serially measure adipose tissue using these point-of-care ultrasound techniques may provide valuable insights into the dynamic changes in body composition occurring in critical illness and help uncover the true relationship between obesity and outcomes in the critically ill.
REFERENCES
- Langouche L, Perre SV, Thiessen S, et al. Alterations in adipose tissue during critical illness: An adaptive and protective response?. Am J Respir Crit Care Med. 2010;182(4):507–516. doi:10.1164/rccm.200909-1395OC
- Jernås M, Olsson B, Sjöholm K, et al. Changes in adipose tissue gene expression and plasma levels of adipokines and acute-phase proteins in patients with critical illness. Metabolism. 2009;58(1):102–108. doi:10.1016/j.metabol.2008.08.012
- Marques MB, Langouche L. Endocrine, metabolic, and morphologic alterations of adipose tissue during critical illness. Crit Care Med. 2013;41(1):317–325. doi:10.1097/CCM.0b013e318265f21c
- Pescatori LC, Savarino E, Mauri G, et al. Quantification of visceral adipose tissue by computed tomography and magnetic resonance imaging: reproducibility and accuracy. Radiol Bras. 2019;52(1):1–6. doi:10.1590/0100-3984.2017.0211
- Starekova J, Hernando D, Pickhardt PJ, Reeder SB. Quantification of liver fat content with CT and MRI: State of the Art. Radiology. 2021;301(2):250–262. doi:10.1148/radiol.2021204288
- Shen W, Wang Z, Punyanita M, et al. Adipose tissue quantification by imaging methods: a proposed classification. Obes Res. 2003;11(1):5–16. doi:10.1038/oby.2003.3
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548–2556. doi:10.1210/jc.2004-0395
- Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12(9):755–767. doi:10.1080/17476348.2018.1506331
- Csige I, Ujvárosy D, Szabó Z, et al. The impact of obesity on the cardiovascular system. J Diabetes Res. 2018;2018:3407306. doi:10.1155/2018/3407306
- Cirulli ET, Guo L, Leon Swisher C, et al. Profound perturbation of the metabolome in obesity is associated with health risk. Cell Metab. 2019;29(2):488–500.e2. doi:10.1016/j.cmet.2018.09.022
- Parameswaran K, Todd DC, Soth M. Altered respiratory physiology in obesity. Can Respir J. 2006;13(4):203–210. doi:10.1155/2006/834786
- Bime C, Fiero M, Lu Z, et al. High positive end-expiratory pressure is associated with improved survival in obese patients with acute respiratory distress syndrome. Am J Med. 2017;130(2):207–213. doi:10.1016/j.amjmed.2016.09.029
- Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116(11):1234–1241. doi:10.1161/CIRCULATIONAHA.107.710509
- Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69(1):29–35. doi: 10.1016/j.diabres.2004.11.007
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi:10.1146/annurev-immunol-031210-101322
- Visaria A, Setoguchi S. Body mass index and all-cause mortality in a 21st century U.S. population: A National Health Interview Survey analysis. PLoS One. 2023;18(7): e0287218. doi: 10.1371/journal.pone.0287218
- Bai L, Huang J, Wang D, et al. Association of body mass index with mortality of sepsis or septic shock: an updated meta-analysis. J Intensive Care. 2023;11(1):27. doi:10.1186/s40560-023-00677-0
- Ni YN, Luo J, Yu H, et al. Can body mass index predict clinical outcomes for patients with acute lung injury/acute respiratory distress syndrome? A meta-analysis. Crit Care. 2017;21(1):36. doi:10.1186/s13054-017-1615-3
- Oliveros H, Villamor E. Obesity and mortality in critically ill adults: a systematic review and meta-analysis. Obesity (Silver Spring). 2008;16(3):515–521. doi:10.1038/oby.2007.102
- Li S, Zhang W, Fu Z, Liu H. Impact of obesity on all-cause and cause-specific mortality among critically ill men and women: a cohort study on the eICU database. Front Nutr. 2023;10:1143404. doi:10.3389/fnut.2023.1143404
- Ju S, Lee TW, Yoo JW, et al. Body Mass Index as a Predictor of Acute Kidney Injury in Critically Ill Patients: A Retrospective Single-Center Study. Tuberc Respir Dis (Seoul). 2018;81(4):311–318. doi:10.4046/trd.2017.0081
- Druml W, Metnitz B, Schaden E, et al. Impact of body mass on incidence and prognosis of acute kidney injury requiring renal replacement therapy. Intensive Care Med. 2010;36(7):1221–1228. doi:10.1007/s00134-010-1844-2
- Gong MN, Bajwa EK, Thompson BT, Christiani DC. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax. 2010;65(1):44–50. doi:10.1136/thx.2009.117572
- Buetti N, Souweine B, Mermel L, et al. Obesity and risk of catheter-related infections in the ICU. A post hoc analysis of four large randomized controlled trials. Intensive Care Med. 2021;47(4):435–443. doi:10.1007/s00134-020-06336-4
- Dickerson RN, Andromalos L, Brown JC, et al. Obesity and critical care nutrition: current practice gaps and directions for future research [published correction appears in Crit Care. 2023 May 8;27(1):177]. Crit Care. 2022;26(1):283. doi:10.1186/s13054-022-04148-0
- Robinson MK, Mogensen KM, Casey JD, et al. The relationship among obesity, nutritional status, and mortality in the critically ill. Crit Care Med. 2015;43(1):87–100. doi:10.1097/CCM.0000000000000602
- Wirtz TH, Loosen SH, Schulze-Hagen M, et al. CT-based determination of excessive visceral adipose tissue is associated with an impaired survival in critically ill patients. PLoS One. 2021;16(4): e0250321. doi:10.1371/journal.pone.0250321
- Rossi AP, Gottin L, Donadello K, et al. Intermuscular adipose tissue as a risk factor for mortality and muscle injury in critically ill patients affected by COVID-19. Front Physiol. 2021;12:651167. doi:10.3389/fphys.2021.651167
- Rossi AP, Donadello K, Schweiger V, et al. Epicardial adipose tissue volume and CT-attenuation as prognostic factors for pulmonary embolism and mortality in critically ill patients affected by COVID-19. Eur J Clin Nutr. 2023;77(1):105–111. doi:10.1038/s41430-022-01197-0
- Alja’nini Z, McNelis KM, Viswanathan S, et al. Infant body composition assessment in the neonatal intensive care unit (NICU) using air displacement plethysmography: Strategies for implementation into clinical workflow. Clin Nutr ESPEN. 2021;43:212–222. doi:10.1016/j.clnesp.2021.04.014
- Mitsides N, McHugh D, Swiecicka A, et al. Extracellular resistance is sensitive to tissue sodium status; implications for bioimpedance-derived fluid volume parameters in chronic kidney disease. J Nephrol. 2020;33(1):119–127. doi:10.1007/s40620-019-00620-3
- Denneman N, Hessels L, Broens B, et al. Fluid balance and phase angle as assessed by bioelectrical impedance analysis in critically ill patients: a multicenter prospective cohort study. Eur J Clin Nutr. 2020;74(10):1410–1419. doi:10.1038/s41430-020-0622-7
- Seidell JC, Bakker CJ, van der Kooy K. Imaging techniques for measuring adipose-tissue distribution–a comparison between computed tomography and 1.5-T magnetic resonance. Am J Clin Nutr. 1990;51(6):953–957. doi:10.1093/ajcn/51.6.953
- Irlbeck T, Massaro JM, Bamberg F, et al. Association between single-slice measurements of visceral and abdominal subcutaneous adipose tissue with volumetric measurements: the Framingham Heart Study. Int J Obes (Lond). 2010;34(4):781–787. doi:10.1038/ijo.2009.279
- Bullen BA, Quaade F, Olessen E, Lund SA. Ultrasonic reflections used for measuring subcutaneous fat in humans. Hum Biol. 1965;37(4):375–384.
- Müller W, Lohman TG, Stewart AD, et al. Subcutaneous fat patterning in athletes: selection of appropriate sites and standardisation of a novel ultrasound measurement technique: ad hoc working group on body composition, health and performance, under the auspices of the IOC Medical Commission. Br J Sports Med. 2016;50(1):45–54. doi:10.1136/bjsports-2015-095641
- Störchle P, Müller W, Sengeis M, et al. Standardized ultrasound measurement of subcutaneous fat patterning: high reliability and accuracy in groups ranging from lean to obese. Ultrasound Med Biol. 2017;43(2):427–438. doi:10.1016/j.ultrasmedbio.2016.09.014
- Suzuki R, Watanabe S, Hirai Y, et al. Abdominal wall fat index, estimated by ultrasonography, for assessment of the ratio of visceral fat to subcutaneous fat in the abdomen. Am J Med. 1993;95(3):309–314. doi:10.1016/0002-9343(93)90284-v
- Bazzocchi A, Filonzi G, Ponti F, et al. Accuracy, reproducibility and repeatability of ultrasonography in the assessment of abdominal adiposity. Acad Radiol. 2011;18(9):1133–1143. doi:10.1016/j.acra.2011.04.014
- Armellini F, Zamboni M, Rigo L, et al. The contribution of sonography to the measurement of intra-abdominal fat. J Clin Ultrasound. 1990;18(7):563–567. doi:10.1002/jcu.1870180707
- Ribeiro-Filho FF, Faria AN, Kohlmann O Jr, et al. Ultrasonography for the evaluation of visceral fat and cardiovascular risk. Hypertension. 2001;38(3 Pt 2):713–717. doi: 10.1161/01.hyp.38.3.713
- Mauad FM, Chagas-Neto FA, Garcia Saab Benedeti AC, et al. Reproducibility of abdominal fat assessment by ultrasound and computed tomography. Radiol Bras. 2017;50(3):141–147. doi:10.1590/0100-3984.2016.0023
- Pontiroli AE, Pizzocri P, Giacomelli M, et al. Ultrasound measurement of visceral and subcutaneous fat in morbidly obese patients before and after laparoscopic adjustable gastric banding: comparison with computerized tomography and with anthropometric measurements. Obes Surg. 2002;12(5):648–651. doi:10.1381/096089202321019620
- Schejbal V. Epikardiales Fettgewebe der rechten Herzkammer–Morphologie, Morphometrie und funktionelle Bedeutung [Epicardial fatty tissue of the right ventricle–morphology, morphometry and functional significance]. Pneumologie. 1989;43(9):490–499.
- Iacobellis G, Ribaudo MC, Assael F, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88(11):5163–5168. doi:10.1210/jc.2003-030698
- Talman AH, Psaltis PJ, Cameron JD, et al. Epicardial adipose tissue: far more than a fat depot. Cardiovasc Diagn Ther. 2014;4(6):416–429. doi: 10.3978/j.issn.2223-3652.2014.11.05
- Bihan H, Heidar R, Beloeuvre A, et al. Epicardial adipose tissue and severe Coronavirus Disease 19. Cardiovasc Diabetol. 2021;20(1):147. doi:10.1186/s12933-021-01329-z
Article citation: York J, Remmert N, Nugent K. Abdominal ultrasonography as a tool to quantify adipose tissue in critically ill patients. The Southwest Respiratory and Critical Care Chronicles 2024;12(51): 1–9
From: Department of Internal Medicine, Texas Tech University Health Sciences Center, Lubbock, Texas
Submitted: 3/3/2024
Accepted: 4/9/2024
Conflicts of interest: none
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.