Abstract
Nafcillin-induced thrombocytopenia: An uncommon complication
Zain Amar MD, Muneeb Rehman MBBS, Alfredo Iardino MD, Yasir Ahmed MD
Corresponding author: Yasir Ahmed
Contact Information: Yasir.Ahmed@ascension.org
DOI: 10.12746/swrccc.v12i52.1305
ABSTRACT
Drug-induced thrombocytopenia is a challenging clinical dilemma that is often overlooked. Nafcillin is a beta-lactam anti-staphylococcal penicillin antibiotic used as a first-line treatment for methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia and severe infections. Nafcillin has been associated with a higher rate of premature antibiotic discontinuation than cefazolin. Here we report a 58-year-old woman with multiple comorbid conditions who presented with a prosthetic right hip joint infection due to MSSA and was treated with nafcillin but developed profound thrombocytopenia due to a possible nafcillin side effect on the 14th day of therapy. Thrombocytopenia resolved after discontinuation of nafcillin, and the patient was treated successfully with cefazolin.
Keywords: Nafcillin-induced thrombocytopenia, drug-induced thrombocytopenia, thrombocytopenia
INTRODUCTION
The incidence of drug-induced thrombocytopenia is not well documented but is estimated at a minimum of 10 cases per one million per year. The actual incidence varies according to the offending drug.1 Nafcillin is a beta-lactam anti-staphylococcal penicillin antibiotic that has been associated with a higher rate of early antibiotic discontinuation due to 18–33.8% adverse effects compared with 2–6.7% for cefazolin.2 Nafcillin rarely causes severe thrombocytopenia with only a few cases previously reported in the literature. The diagnosis requires a temporal relationship between the drug administration and the onset of thrombocytopenia, exclusion of other common causes of thrombocytopenia, and re-challenging with the culprit drug. Here we present a rare case of nafcillin-induced profound thrombocytopenia that resolved after discontinuation of nafcillin.
CASE
A 58-year-old woman with a history of hypertension and severe osteoarthritis had a right hip arthroplasty that was complicated by early osteomyelitis of the proximal right femur at the site of the prosthesis. She underwent step one of the two-stage hip revision with an explantation of all the hardware and placement of the articulating spacer. She was started on empiric IV vancomycin 15 mg/kg, piperacillin-tazobactam 3.375 gm every 6 hours, and enoxaparin 40 mg subcutaneously for deep venous thrombosis prophylaxis. An operative culture confirmed methicillin sensitive Staphylococcus aureus, and antibiotics were switched to nafcillin 12 gm IV continuous infusion over 24 hours via a peripherally inserted central catheter. The platelet count on the day she started on nafcillin therapy was 395 × 103/μL. The repeat platelet count on nafcillin therapy day 3 was 503 × 103/μL. Later, she went home on the nafcillin therapy for 8 weeks.
Repeat laboratory tests showed the platelet count had dropped to 18 × 103/μL on nafcillin day 13 therapy, and she was readmitted immediately. She denied fever, chills, epistaxis, and melena. She had no family history of thrombocytopenia or bleeding disorders. She was taking her regular home medications with no new medication except for nafcillin. On admission, she was afebrile, and her blood pressure was 147/83 mmHg. Physical examination showed bilateral lower extremity purpuric rash extending from ankles to mid-thigh. Admission laboratory examination revealed profound thrombocytopenia with a platelet count of only 1 × 103/μL on nafcillin day 14 (hospital day 1), and other laboratory data are reported in Table 1. A peripheral blood smear showed severe thrombocytopenia without platelet clumping; no schistocytes, helmet cells, or blast cells were seen. She received 1 unit of single donor platelet transfusion, and nafcillin was discontinued with the initiation of cefazolin 2 gm IV every 8 hours. All her home medications were resumed. Intravenous methylprednisolone was also started for suspected idiopathic thrombocytopenia and was discontinued after 48 hours. The patient platelet count improved to 41 × 103/μL on hospital day 2 and normalized to 189 × 103/μL on hospital day 5 after discontinuation of nafcillin. During the hospital course, the purpuric rash started to resolve. The heparin-PF4 antibody was positive, but the serotonin release assay was negative (<10 ng/ml), ruling out heparin-induced thrombocytopenia. The platelet count at the time of discharge was 305 × 103/μL on hospital day 7 after discontinuation of nafcillin (Figure1).
Table 1. Admission and Discharge Laboratory Results
| Study |
Admission Data |
Discharge Data |
Normal Values |
| White Blood Cell, ×103/μL |
5.2 |
7.8 |
4.8–10.8 |
| Hemoglobin, g/dL |
12.5 |
9.3 |
12–16 |
| Hematocrit, % |
34.3 |
29.7 |
37–47 |
| Platelet Count, ×103/μL |
1 |
305 |
130–450 |
| Prothrombin Time seconds |
16.4 |
– |
12.2–14.9 |
| INR |
1.27 |
– |
<1.5 |
| Activated PTT seconds |
38.6 |
– |
23.2–37.4 |
| D.Dimer, mg/L |
0.4 |
|
<0.5 |
| Serum Fibrinogen, mg/dL |
398 |
– |
200–456 |
| Serum Haptoglobin, mg/dL |
123 |
– |
30–200 |
| Fibrin Split Products |
Negative |
|
Negative |
| Blood Urea Nitrogen, mg/dL |
9 |
15 |
7–20 |
| Serum Creatinine, mg/dL |
0.6 |
0.7 |
0.6–1.2 |
| AST, units/L |
17 |
– |
3–38 |
| ALT, units/L |
13 |
– |
<49 |
| Hepatitis C Antibody |
Negative |
– |
Negative |
| HIV Antibody |
Negative |
– |
Negative |
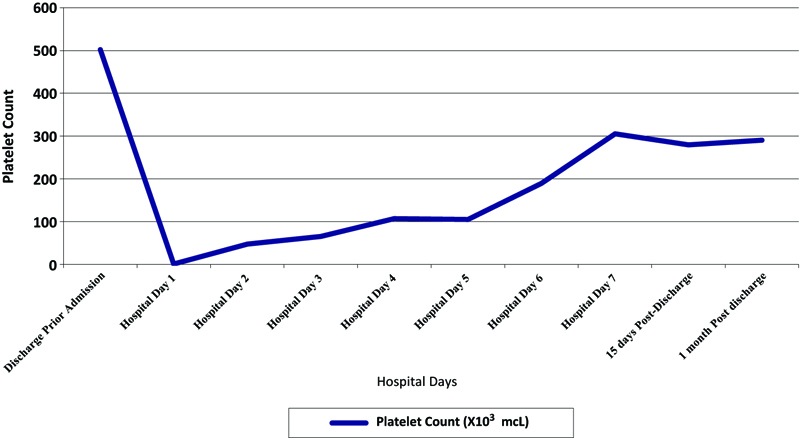
Figure 1. Platelet count trend from first discharge to 1-month post-discharge, showing sharp recovery of platelet count after discontinuation of therapy that was maintained after discharge.
Re-challenge with nafcillin was not done due to severe thrombocytopenia at her presentation. Due to the rapid recovery of platelet count after the discontinuation of the nafcillin drug, a nafcillin-dependent antibody test was not ordered. Subsequently, the patient was discharged home on IV cefazolin 2 gm every 8 hours of therapy to finish the remaining 8 week course of therapy. She successfully finished 8 weeks of antibiotic therapy with no recurrence of thrombocytopenia.
DISCUSSION
Drug-induced thrombocytopenia is, in most cases, autoimmune in etiology and is included in the spectrum of drug-induced immune-mediated thrombocytopenia (DITP); exceptions, such as chemotherapy and other direct cytotoxic medications, exist, but those represent a small percentage of the cases. The onset of drug-induced thrombocytopenia is usually seen after seven to fourteen days of therapy. However, in patients with prior exposure, it can occur earlier in 1 to 3 days.1,3 Our patient developed profound thrombocytopenia on the 14th day of nafcillin therapy.
Myelosuppression, including neutropenia, anemia, and less commonly, thrombocytopenia, is a well-documented serious side effect of nafcillin. A study in adults reported higher rates of neutropenia with nafcillin (8.4%) compared to cefazolin (3.3%), but this difference was not statistically significant.4 The mechanism of drug-induced thrombocytopenia is complex and variable. The hapten-dependent antibody is the most common mechanism of action of thrombocytopenia for penicillin and some of the cephalosporins, characterized by the drug binding covalently to the platelet membrane proteins, causing antibody production and drug-specific immune reaction, which differs from the thrombocytopenia observed with other antibodies, such as β-lactam antibiotics, vancomycin, sulfonamide, rifampin, fluoroquinolone, pentamidine, and linezolid, that is triggered by a quinine-type antibody. In these cases, the drug induces the production of the antibody that later will attach to the platelet and destroy the membrane, which is the mechanism by which nafcillin could induce destruction of platelets.1,5 Understanding the mechanism of drug induced platelet dysfunction or lysis will dictate the treatment. The discontinuation of the offending drug is the essential step in treatment, but the use of corticosteroids, although common, has not been determined to be effective. After the drug is cleared out of the patient’s system and the use of a pharmacologically equivalent drug with a different chemical structure has begun, the platelet count starts to rise, usually after 48 hours.6
Our patient was readmitted to the hospital after 14 days of nafcillin continuous infusion with a platelet count of 1,000/µL. Initial differential diagnosis included heparin-induced thrombocytopenia (HIT), DITP, and less likely DIC in the presence of normal coagulation studies. Nafcillin was immediately stopped, and cefazolin 2 gram IV every 8 hours was initiated, and it was an equivalent drug with a different chemical structure.2,7,8
When evaluating a similar patient, a systematic approach and differential diagnosis need to be established; not all institutions have available specific assays for drug-dependent antibodies, and diagnosis by exclusion or empirical diagnosis in those cases needs to be made.9 In 2005, George and colleagues described a model to determine criteria and level of evidence for establishing a causative relationship in drug-induced thrombocytopenia (Table 2), and our case meets 3 out of 4 criteria since we could not re-challenge the patient with nafcillin based on the patient’s request and establishes level II of evidence (Probable) of nafcillin being the trigger for the patient thrombocytopenia.10
Table 2. Criteria and Level of Evidence for Establishing a Causative Relationship in Drug-induced Thrombocytopenia
| Criterion |
Description |
| 1 |
Therapy with the candidate drug preceded thrombocytopenia, and recovery from thrombocytopenia was complete and sustained after discontinuation of therapy |
| 2 |
The candidate drug was the only drug used before the onset of thrombocytopenia, or other drugs were continued or re-introduced after discontinuation of therapy with the candidate drug, with a sustained normal platelet count. |
| 3 |
Other causes of thrombocytopenia were ruled out |
| 4 |
Re-exposure to the candidate drug resulted in recurrent thrombocytopenia |
| Level of Evidence |
Description |
| I |
Definite – Criteria 1, 2, 3, and 4 are met. |
| II |
Probable – Criteria 1, 2, and 3 are met. |
| III |
Possible – Criteria 1 is met. |
| IV |
Unlikely – Criteria 1 is not met. |
In summary, drug-induced thrombocytopenia is always a challenging clinical dilemma that is often overlooked. Nafcillin, even if few reports are available, can cause severe thrombocytopenia, and the discontinuation of therapy is the essential decision. The diagnosis requires a temporal relationship between the drug administration and the onset of thrombocytopenia and exclusion of other causes of thrombocytopenia. The patient who requires long term IV nafcillin therapy should have a regular complete blood count and chemistry, at least weekly, to monitor this rare side effect of thrombocytopenia apart from leukopenia and nephrotoxicity.
REFERENCES
- Loo AS, Gerzenshtein L, Ison MG. Antimicrobial drug-induced thrombocytopenia: a review of the literature. Semin Thromb Hemost 2012;38:818–29.
- Flynt LK, Kenney RM, Zervos MJ, et al. The safety and economic impact of cefazolin versus nafcillin for the treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections. Infect Dis Ther 2017;6(1):225–31.
- Papadakis MA, McPhee SJ. 2016 CURRENT Medical Diagnosis & Treatment, 55 ed. United States of America: McGraw-Hill Education; 2016.
- Youngster I, Shenoy ES, Hooper DC, et al. Comparative evaluation of the tolerability of cefazolin and nafcillin for treatment of methicillin-susceptible Staphylococcus aureus infections in the outpatient setting. Clin Infect Dis 2014;59: 369–75.
- Ahmed Y, Sartin C, Umer I, et al. Vancomycin-induced immune thrombocytopenia. The Southwest Respiratory and Critical Care Chronicles 2015;3(9):42–45.
- Aster RH, Curtis BR, McFarland JG, et al. Drug-Induced Immune thrombocytopenia: pathogenesis, diagnosis, and management. J Thromb Haemost 2009;7:911–18.
- Monogue ML, Ortwine JK, Wei W, et al. Nafcillin versus cefazolin for the treatment of methicillin-susceptible Staphylococcus aureus bacteremia. J Infect Public Health 2018 Sep–Oct;11(5):727–73.
- Rindone JP, Mellen CK. Meta-analysis of trials comparing cafazolin to antistaphylococcal penicillins in the treatment of methicillin-sensitive Staphylococcus aureus bacteremia. Br J Clin Pharmacol 2018;84(6):1258–66.
- Visentin GP, Liu CY. Drug-induced thrombocytopenia. Hematol Oncol Clin North Am 2007;21(4):685–96, vi.
- Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med 2007;357:580–87.
Article citation: Amar Z, Rehman M, Iardino A, Ahmed Y. Nafcillin-induced thrombocytopenia: An uncommon complication. The Southwest Respiratory and Critical Care Chronicles 2024;12(52):20–24
From: Infectious Diseases Department (ZA, MR, YA), Ascension St. John Medical Center, Tulsa, OK; CHI St. Alexius Medical Center (AI), Bismarck, ND
Submitted: 4/15/2024
Accepted: 6/26/2024
Conflicts of interest: none
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.