Abstract
The impact of intravenous metoprolol tartrate on mortality rates in patients with septic shock due to ventilator-associated pneumonia: A randomized clinical trial
Akram Muhammad Fayed MD, Haytham Saber Meligy MD, Sherouk Rafik Hamad MD
Corresponding author: Sherouk Rafik Hamad
Contact Information: Sheroukrafik@gmail.com
DOI: 10.12746/swrccc.v12i53.1351
ABSTRACT
Background: Septic shock, particularly due to ventilator-associated pneumonia (VAP), is a significant cause of mortality in critically ill patients. Traditional treatments include antimicrobial therapy, fluid resuscitation, and vasopressors. However, recent interest has emerged in using β-blockers to modulate the hyperadrenergic state seen in sepsis. β-blockers, like metoprolol, may improve cardiac efficiency, reduce myocardial oxygen demand, and ultimately enhance patient outcomes.
Objective: This study aimed to assess the impact of administering intravenous metoprolol tartrate on the mortality rates of patients with septic shock due to VAP.
Methods: This study employed a randomized clinical trial design within the Intensive Care Units of Alexandria Main University Hospital. A cohort of 100 patients diagnosed with septic shock due to ventilator-associated pneumonia (VAP) participated in the trial. Upon achieving hemodynamic stabilization, participants were randomly assigned to either receive standard care alone or standard care supplemented with intravenous metoprolol. Variables of interest included assessing 28-day mortality rates, duration of ICU stay and mechanical ventilation, reliance on intravenous fluids and vasopressors, acid-base balance, lactate levels, inflammatory markers, and mean arterial pressure (MAP). This comprehensive approach aimed to evaluate the impact of metoprolol on critical outcomes in this patient population.
Results: The administration of intravenous metoprolol tartrate resulted in significant clinical benefits, including lower 28-day mortality rates (P = 0.013), shortened durations of ICU stay (P < 0.001), and reduced mechanical ventilation periods (P < 0.001). These outcomes were explained by the observed decreased reliance on intravenous fluids and vasopressors (P < 0.001), improved acid-base balance and lactate levels. The rapid reduction of inflammatory markers (P < 0.001) and the sustained improvement of MAP in the metoprolol group compared to the control group further contributed to the explication of these findings.
Conclusion: Intravenous metoprolol tartrate effectively controlled inflammation, optimized hemodynamics, and improved patient outcomes compared to standard care, suggesting it as a beneficial adjunct in the management of septic shock.
Trial registration: PACTR202404531355476.
Keywords: Metoprolol; septic shock; ventilator-associated pneumonia; beta-blockers; mortality.
INTRODUCTION
Sepsis and septic shock are significant challenges in global healthcare, annually impacting millions and causing considerable mortality.1 The sepsis term indicates life-threatening organ dysfunction arising from the body’s dysregulated response to infection; septic shock represents a severe state characterized by circulatory and cellular irregularities, significantly increasing mortality risks.2,3 Pneumonia not only leads to most sepsis cases in ICU patients, but it also complicates ventilator management. Ventilator-associated pneumonia (VAP) affects approximately one third of mechanically ventilated patients and has a 4.6% mortality rate.4,5
Symptoms of septic shock start with fever, rapid heart rate, and decreased alertness, progressing to low blood pressure, cool extremities, and organ-specific dysfunction.6 Managing septic shock involves prompt resuscitation, the initiation of antimicrobials within one hour of recognition, hemodynamic stabilization, ventilation strategies, and judicious use of additional therapies.2,7 Excessive adrenergic response in septic shock causes a cascade of physiological changes, which impact immune function,8 thrombogenicity,9 metabolic efficiency,10 and cardiac function.11–13 High plasma catecholamine levels correlate with poor outcomes.14
Beta-blockers like metoprolol,15 despite concerns,16 offer potential benefits in managing septic shock by mitigating the adrenergic surge,17,18 improving cardiac and pulmonary function,19,20 and modulating the inflammatory response.21 It was hypothesized that achieving heart rate control to a specified limit using intravenous metoprolol tartrate in patients with septic shock due to VAP would confer significant clinical benefits. Reducing heart rate should increase diastolic filling time, thereby increasing cardiac diastolic filling and overall hemodynamic stability. This improvement was expected to positively affect patient outcomes, including 28-day mortality rates. In addition, the study monitored duration of mechanical ventilation, length of ICU stays, and perfusion parameters to evaluate the overall impact of metoprolol administration.
METHODS
This randomized clinical trial was conducted in the 122-bed general Intensive Care Unit (ICU) at Alexandria Main University Hospital, Alexandria, Egypt. Inclusion criteria included patients with septic shock from VAP, maintaining hemodynamic stability (consistent mean arterial pressure [MAP] ≥ 65 mmHg and unchanged vasopressor dosages for ≥3 hours), preserved left ventricular ejection function (LVEF > 45% without significant valvular abnormalities), adequate sedation (Richmond Agitation–Sedation Scale score 0 to −3), and heart rate >100 beats per minute. Ventilator-associated pneumonia was identified using guidelines provided by the American Thoracic Society and Infectious Diseases Society of America.22 Exclusion criteria included pregnancy, chronic beta-blocker use, and beta-blocker contraindications.
Patients were randomized to receive either conventional management or conventional management plus intravenous metoprolol tartrate (Metopronad, Inad Pharma, Giza, Egypt). Metoprolol was not administered as a continuous maintenance dose; instead, it was given on an as-needed basis in response to elevated heart rates. When a patient’s heart rate exceeded 80 beats per minute, metoprolol tartrate was administered in increments of 2.5–5 mg intravenously with doses repeated every 2–5 minutes as necessary. The total cumulative dose was limited to a maximum of 15 mg over 10–15 minutes. The goal of this dosing regimen was to achieve a target heart rate of 70–80 beats per minute. No routine or maintenance dosing was provided; metoprolol was administered only in response to heart rate elevations above the specified threshold. The administration was discontinued if mean arterial pressure fell below 65 mmHg, heart rate deviated from the target range, or severe arrhythmias developed.
Standard treatment protocols followed surviving sepsis guidelines, with antibiotic administration guided by treating clinicians. Arterial and central venous blood samples were analyzed for blood gas parameters, lactate levels, haematology, biochemistry, kidney and liver function, coagulation profile, and inflammatory markers, such as procalcitonin and C-reactive protein. Baseline and post-randomization data included hemodynamic measurements, laboratory variables, blood gas analyses, and norepinephrine requirements, and adverse events.
STATISTICAL ANALYSIS
Statistical analyses were conducted using Stata® software version 16 (StataCorp LLC, Texas, USA), with all hypotheses constructed as two-tailed tests and significance set at p ≤ 0.05. The Shapiro–Wilk test was used to assess the normality of data, revealing a non-parametric distribution. Continuous variables were reported as medians with interquartile ranges (IQR), while categorical variables were reported as frequencies and percentages. Comparisons of categorical variables were performed using the Chi-square test or Fisher exact test as appropriate. Pairwise comparisons of continuous variables were conducted using the Mann-Whitney test. The Friedman test, serving as a non-parametric alternative to repeated measures analyses of variance (ANOVA), was employed for comparisons involving multiple measurements.
RESULTS
Patient Recruitment: After achieving hemodynamic optimization, 100 patients were included in the study for primary analysis after exclusion and randomization (Figure 1).
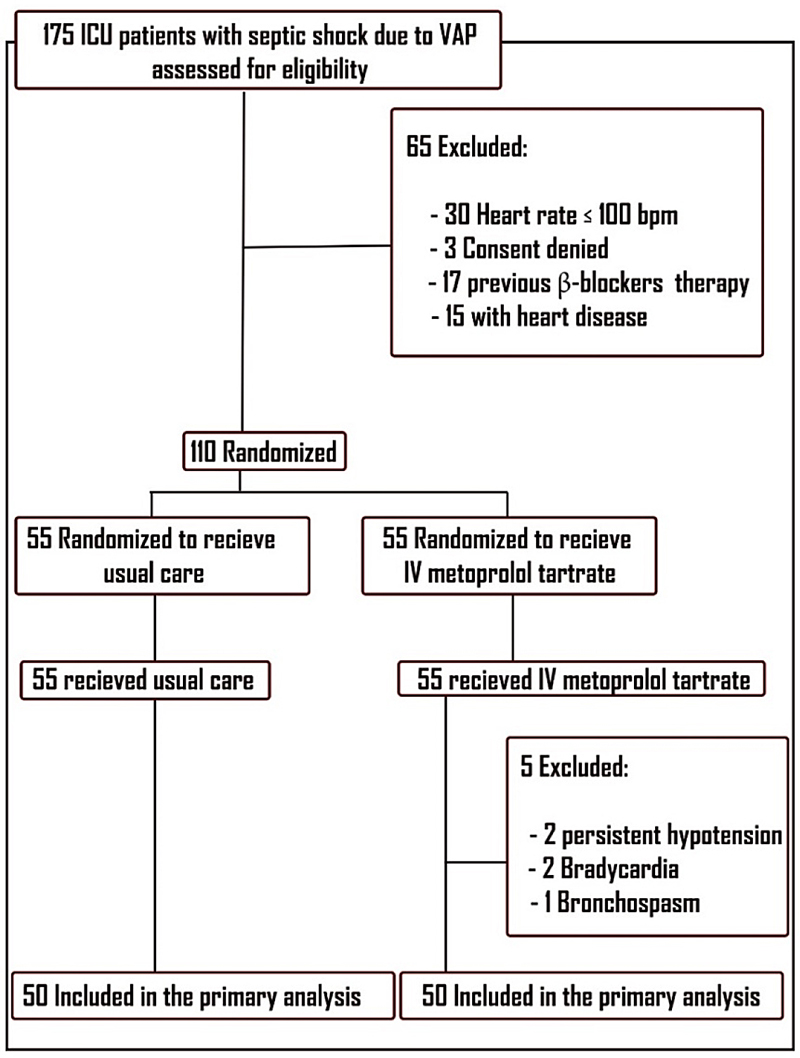
Figure 1. Patients’ enrolment in the study. 175 patients were screened initially for eligibility, excluding 47 with heart rates below the target or prior β-blocker therapy, three due to unattainable informed consent, and 15 with valvular heart diseases. This left 110 patients, randomly assigned to the study groups in a 1:1 ratio. Five patients from the metoprolol group were excluded due to complications, resulting in 100 patients for primary analysis.
Demographic data: Parameters, including age, sex, APACHE II score, SOFA score, hypoxic index, comorbidities, and pathogen spectrum, demonstrated harmonious distribution among the study groups, ensuring a robust foundation for comparative analysis. The time from shock onset to randomization was similar between groups, indicating no significant timing bias (Table 1).
Table 1. Patients’ Baseline Characteristics
|
Control Group |
Metoprolol Group |
P |
| A. Demographics |
| Age, years |
61 (55–66.7) |
60 (55–65) |
0.607 |
| Males, (No.), % |
25 (51.1%) |
25 (50%) |
0.841 |
| Females, (No.), % |
24 (48.9%) |
25 (50%) |
| B. Disease Severity |
| APACHE II score |
22 (19–24) |
22.5 (20–24) |
0.100 |
| SOFA score |
15 (13–16.5) |
14 (13–17) |
0.633 |
| Time since the onset of shock state to randomization (hours) |
24 (24–36) |
25 (20–36) |
0.843 |
| C. Associated co-morbidities, No. (%) |
| Hypertension |
35 (70%) |
29 (58%) |
0.265 |
| Diabetes mellitus |
20 (40%) |
30 (60%) |
0.063 |
| Cerebrovascular events |
0 |
3 (6%) |
0.186 |
| Chronic kidney disease |
3 (6%) |
5 (10%) |
0.413 |
| Chronic obstructive pulmonary disease |
6 (12%) |
9 (18%) |
0.420 |
| Endocrinal diseases |
1 (2%) |
4 (8%) |
0.338 |
| Others |
5 (10%) |
8 (16%) |
0.776 |
| D. Pathogens, No. (%) |
| Pseudomonas spp |
15 (30%) |
11 (22%) |
0.912 |
| Klebsiella spp |
10 (20%) |
14 (28.5%) |
0.795 |
| Acinetobacter spp |
13 (26%) |
16 (32.6%) |
0.863 |
| Escherichia Coli |
3 (6%) |
2 (4%) |
0.607 |
| Staphylococcus aureus |
4 (8%) |
5 (10%) |
0.728 |
| Others |
12 (24%) |
7 (14%) |
0.677 |
| E. Vital signs |
| Heart Rate |
133 (113–141.5) |
130.5 (117.5–138.2) |
0.939 |
| Mean arterial pressure (mmHg) |
67 (65–69) |
65.5 (65–68) |
0.509 |
| Respiratory rate |
24 (20–27) |
24 (21–26) |
0.868 |
| Temperature (°C) |
38.5 (37.8–39.3) |
38.5 (37.8–39.2) |
0.771 |
| F. Hematological data |
| Hemoglobin (g/dl) |
11.3 (10.5–11.9) |
11.4 (10.6–12.4) |
0.359 |
| WBCs (×103/cmm) |
23.5 (21–23.5) |
22.7 (21–28.7) |
0.143 |
| G. Kidney functions & Electrolytes |
| Urea (mg/dl) |
124.5 (114–132) |
124.5 (113.7–137.7) |
0.091 |
| Creatinine (mg/dl) |
2.5 (1.2–5.6) |
2.4 (1.6–5.3) |
0.964 |
| Sodium (mmol/l) |
136 (134–139) |
136 (133.7–139) |
0.806 |
| Potassium (mmol/l) |
4 (3.9–4.3) |
4.1 (3.8–4.3) |
0.862 |
| H. Arterial blood gases |
| pH |
7.3 (7.29–7.34) |
7.3 (7.29–7.33) |
0.106 |
| PaCo2 (mmHg) |
36.9 (31.7–39.5) |
38.8 (34.5–42.3) |
0.068 |
| PaO2 (mmHg) |
74.04 (70–77.9) |
75.07 (70.5–81) |
0.49 |
| HCO3 (mmHg) |
17.6 (14–19) |
16.9 (15.4–19.8) |
0.912 |
| Hypoxic index |
176.5 (150–200) |
175 (155–200) |
0.893 |
| Lactate (mmol/l) |
10.1 (8.3–12.1) |
10.2 (8.7–12.7) |
0.296 |
| I. Inflammatory Markers |
| C-reactive protein (mg/dl) |
225.5 (198–256.7) |
243.5 (215–265) |
0.0721 |
| Procalcitonin (ng/dl) |
33 (29.15–35.8) |
32.9 (27.82–38.4) |
0.134 |
Study drug dosage stability: Intravenous metoprolol tartrate maintained a stable dosage over time, with a median of 15 mg (IQR, 12.5–20); this did not exceed the specified maximum daily dosage of 15 mg. The stability observed in metoprolol dosage establishes a foundation for its controlled administration, ensuring safety while exploring its therapeutic potential.
Hemodynamic variables and therapies: In adherence to a target heart rate range of 70/min to 80/min, the metoprolol group exhibited consistently lower heart rates throughout the intervention period compared to the control group. The median AUC substantiated this difference, underscoring 76/min (IQR, 75 to 80) for the metoprolol group versus 90/min (IQR, 110 to 125) for the control group (P < .001) (Figures 2, 3).
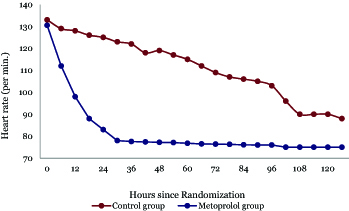
Figure 2. Line graph for the changes of heart rate over time in both groups.
This figure illustrates the temporal changes in HR and MAP in both groups over 120 hours post-randomization. HR in the control group gradually declined from 133 bpm to 90 bpm till reaching a plateau indicating a steady physiological response with improvement in hemodynamics, while metoprolol rapidly reduced HR from 130.5 bpm to around 77 bpm in an average of 36 hours. MAP in controls increased slightly from 67 mmHg to 76 mmHg, contrasting with metoprolol’s marked rise to 92 mmHg.
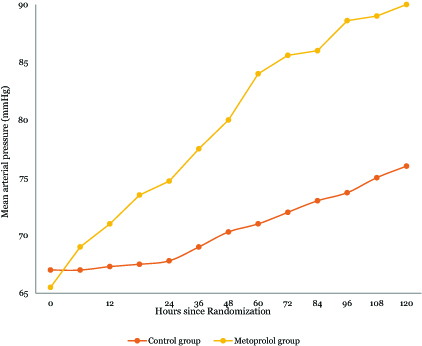
Figure 3. Line graph for the changes of mean arterial pressure over time in both groups.
This figure illustrates the temporal changes in HR and MAP in both groups over 120 hours post-randomization. HR in the control group gradually declined from 133 bpm to 90 bpm till reaching a plateau indicating a steady physiological response with improvement in hemodynamics, while metoprolol rapidly reduced HR from 130.5 bpm to around 77 bpm in an average of 36 hours. MAP in controls increased slightly from 67 mmHg to 76 mmHg, contrasting with metoprolol’s marked rise to 92 mmHg.
Intravenous fluid: Beyond heart rate modulation, the metoprolol group had reduced intravenous fluid requirements with a median of 12.8 L (IQR, 11–14.25) versus 16.7 L (IQR, 16–17.9). The metoprolol group had decreased vasopressor requirement recording a median of 88.6 mg (IQR, 83–97.5) vs 126.5 mg (IQR, 112.8–147.1) for the control group, and it had superior maintenance of MAP.
Inflammatory markers: The control group and the metoprolol group both had significant reductions in C reactive protein levels during follow-up compared to their admission levels. In the control group the median CRP level decreased from 225.5 mg/dl on admission to 79 mg/dl on follow-up; in the metoprolol group CRP decreased from 243.5 mg/dl to 61 mg/dl. The metoprolol group showed a significantly greater decrease in CRP levels compared to the control group at the final assessment (p < 0.001) (Table 2). Both groups had significant reductions in procalcitonin levels after the resolution of the shock state. The control group’s median procalcitonin level decreased from 33 ng/dl initially to 2.3 ng/dl on follow-up, while the metoprolol group’s decreased from 36.2 ng/dl to 1.6 ng/dl. The improvement in procalcitonin levels was significantly better in the metoprolol group compared to the control group (P < 0.001) (Table 2).
Table 2. Laboratory Data After Resolution of Shock
|
Control Group |
Metoprolol Group |
P |
| Hemoglobin (g/dl) |
11.1 (10.3–12.2) |
11.5 (10.7–12.6) |
0.488 |
| WBCs (×103/cmm) |
8.4 (7.6–9.7) |
8.7 (7.5–9.7) |
0.942 |
| Urea (mg/dl) |
42.4 (32.6–47.6) |
43.4 (34.5–51.2) |
0.436 |
| Creatinine (mg/dl) |
1.7 (1.3–2) |
1.6 (1.2–1.8) |
0.385 |
| Sodium (mmol/l) |
138.5 (135.7–141) |
138.5 (135–141) |
0.763 |
| Potassium (mmol/l) |
4.1 (3.9–4.5) |
4.2 (3.9–4.5) |
0.283 |
| Arterial blood gases |
| PH |
7.39 (7.37–7.42) |
7.40 (7.38–7.45) |
0.009** |
| PaCo2 (mmHg) |
41.3 (37.7–43.8) |
43 (40.5–45.3) |
0.025* |
| HCO3 (mmHg) |
23.7 (22.5–24.9) |
26 (24.7–27.4) |
<0.001*** |
| PaO2 (mmHg) |
93.7 (90.7–96.5) |
92.8 (90–95) |
0.316 |
| Lactate (mmol/l) |
1.8 (1.6–1.9) |
1.3 (0.9–1.7) |
<0.001*** |
| Hypoxic index (PaO2/FiO2 ratio) |
209 (192–208) |
207.8 (195.5–211) |
0.786 |
| Inflammatory Markers |
| C-reactive protein (mg/dl) |
79 (74–84.2) |
61 (54.7–67.2) |
<0.001*** |
| Procalcitonin (ng/dl) |
2.3 (1.9–2.7) |
1.6 (1.1–1.9) |
<0.001*** |
Acid-base and metabolic dynamics: Metoprolol administration had notable effects on acid-base balance and metabolic parameters. There were higher median areas under the curve observed for arterial pH and HCO3 and lower median AUC for serum lactate concentration compared to the control group. However, it is important to note that while these differences reached statistical significance, their clinical relevance may be limited, as metabolic variables remained within normal ranges in both groups. Hypoxic index and oxygen saturation remained similar between the two groups (Table 2).
Ventilator parameters: On comparing ventilator parameters on randomization and before weaning, a significant improvement in both control and metoprolol groups was noted (P1 < 0.001). However, this improvement couldn’t have been attributed to metoprolol administration, as there were no significant differences between the groups. The observed improvement is likely due to standard management protocols during treatment (Table 3).
Table 3. Ventilator Settings of Patients at Randomization and at weaning
|
Control Group |
Metoprolol Group |
P |
| Mode of ventilation |
P A-C |
P A-C |
|
| Tidal volume (VT) (ml) |
| At randomization |
520 [498.5–550] |
520 [500–540] |
0.609 |
| At weaning |
560 [543–584] |
572 [553.5–594] |
0.104 |
| P1 |
<0.001*** |
<0.001*** |
|
| Respiratory Rate (breaths/minute) |
| At randomization |
24 [20–27] |
24 [21–26] |
0.868 |
| At weaning |
15 [14–16] |
15 [12–16] |
0.336 |
| P1 |
<0.001*** |
<0.001*** |
|
| Positive End-Expiratory pressure (PEEP) (cmH2O) |
| At randomization |
9 [8–10] |
9 [8–9] |
0.216 |
| At weaning |
5 [5–6] |
5 [5–6] |
0.667 |
| P1 |
<0.001*** |
<0.001*** |
|
| Peak inspiratory pressure (PIP) (cmH2O) |
| At randomization |
29 [26.5–30.5] |
29 [28–30] |
0.606 |
| At weaning |
23 [22.5–26] |
25 [23–27] |
0.119 |
| P1 |
<0.001*** |
<0.001*** |
|
Organ function and injury markers: Metoprolol tartrate administration did not have a significant impact on kidney or liver functions, as indicated by the lack of significant differences between the two groups.
Outcome data: The metoprolol group had a 28-day mortality rate of 49.4%, significantly lower than the control group of 80.5% (P < 0.001). The survival trajectory exhibited by the metoprolol group included a reduction in both the duration of mechanical ventilation and ICU stay. It is noteworthy that metoprolol administration did not reduce in hospital mortality (Figure 4).
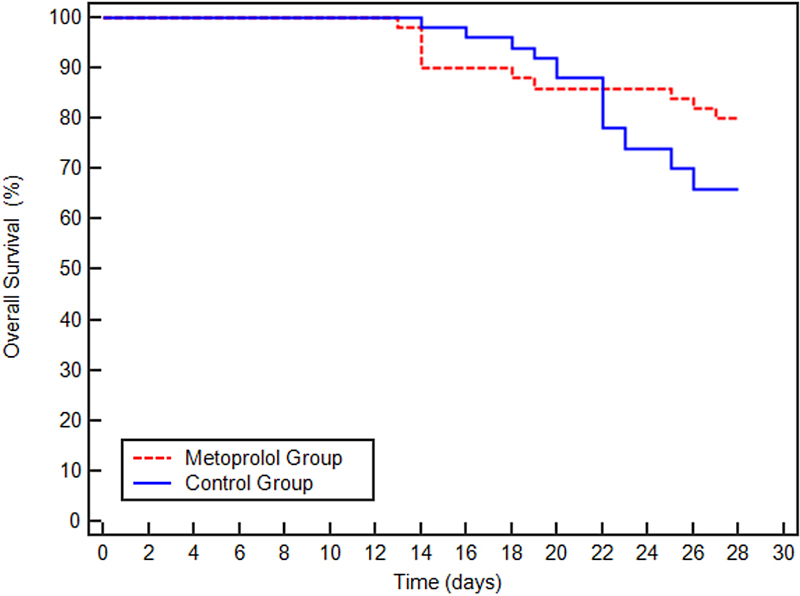
Figure 4. Summary of the study outcomes.
Figure 4 shows the Kaplan-Meier survival curves for patients in the Metoprolol and Control groups over a 30-day period. The survival rate for both groups starts at 100% and declines over time. Notably, the Control group demonstrates a more rapid decline in survival compared to the Metoprolol group, particularly after day 10, which suggests a potential survival benefit for patients receiving metoprolol.
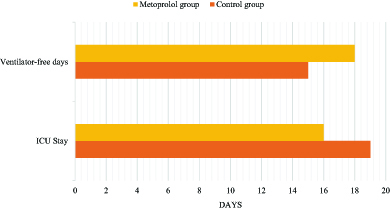
Figure 5. Summary of the study outcomes.
The ICU length of stay was slightly shorter in the metoprolol group 16 days vs 19 days in the control group. Patients in the metoprolol group were liberated earlier from mechanical ventilation.
DISCUSSION
The management of septic shock, a life-threatening condition, has improved due to advances in medical care. Early detection and prompt initiation of antimicrobial therapy alongside advanced life support measures have notably improved patient outcomes during the initial resuscitation phase.2 However, patients remain susceptible to infections within the ICU following successful resuscitation, and VAP is a significant concern.5 Ventilator-associated pneumonia is closely linked to prolonged mechanical ventilation and extended ICU stays. The diagnosis relies on a triad of clinical, microbiological, and radiological criteria.23,24 Prompt administration of appropriate antimicrobial therapy is crucial for limiting its impact on patient outcomes.
The use of β-blockers has received attention in the management of various critical illnesses, including sepsis.16,18 Prior observational studies have suggested a correlation between β-blocker therapy and improved survival rates in critically ill patients.25–27 In addition, early administration of short-acting β-blockers has shown promise in reducing sepsis mortality rates within 28 days.12
The rationale for using β-blockers in septic patients stems from the intricate dynamics of the sympathetic nervous system and the baroreflex responses in sepsis. Initially, during sepsis, the sympathetic nervous system maintains cardiac output and blood pressure by modulating heart rate, contractility, and vascular tone. This compensatory mechanism is reflected in early sepsis-induced tachycardia, which serves to offset decreased stroke volume, indicative of effective baroreflex activity. Moreover, adequate volume resuscitation typically leads to a reduction in heart rate because of the compensatory nature of tachycardia.28,29
However, in septic shock, this delicate balance is often disrupted. Elevated catecholamine levels contribute to a hyperadrenergic state, which can compromise the baroreflex response. This manifests as sustained tachycardia, persisting despite sufficient fluid resuscitation and analgesia, signifying excessive sympathetic stimulation. Notably, persistent tachycardia, even following norepinephrine infusion, is associated with a significantly elevated mortality risk due to depleted compensatory mechanisms. Prolonged tachycardia imposes strain on the heart by increasing oxygen demand, limiting diastolic filling, and eliciting direct cardiotoxic effects, and this underscores the critical need for effective management strategies in septic patients.30
Hence, there is a growing interest in exploring interventions to address tachycardia in septic patients, given its implications for patient outcomes. Our randomized controlled trial involving 100 patients with ventilator-associated pneumonia-induced septic shock and high mortality risk aimed to study this issue. However, addressing tachycardia in septic shock remains complex, while reducing heart rate may interfere with compensatory mechanisms, maintaining excessive tachycardia may compromise organ perfusion.31 Moreover, the optimal timing for intervention and heart rate threshold remains undefined. In our study, we proposed a heart rate range of 70/min to 80/min as a suitable compromise between enhancing cardiac performance and maintaining systemic hemodynamics and limiting excess sympathetic activity, which was safely achieved within the initial 48 hours of treatment.
This study not only revealed promising outcomes but also helps explain the underlying mechanisms contributing to its potential clinical benefits. The precise modulation of heart rate dynamics by metoprolol was translated into tangible improvements in tissue perfusion, as evidenced by lower intravenous fluid requirements and reduced vasopressor consumption. The metoprolol group demonstrated superior MAP maintenance, indicating a positive influence on systemic vascular resistance and cardiac output. The observed anti-inflammatory effect, characterized by reduced procalcitonin and CRP levels, suggests a potential modulation of the immune response, further contributing to hemodynamic stability.
The differences in metabolic variables were statistically significant between the groups and included higher arterial pH and HCO3 levels and a lower serum lactate concentration in the metoprolol group. Notably, despite these observed differences, both groups had values within normal clinical ranges, indicating potential limitations in their clinical relevance.
The substantial clinical impact became evident in the outcome data, with the metoprolol group having a significantly lower 28-day mortality rate than the control group. This enhanced survival was accompanied by a shorter duration of mechanical ventilation and ICU stay, pointing toward a potential role of metoprolol in improving recovery. These findings contribute to the growing discourse on the potential clinical advantages of metoprolol intervention in the context of septic shock, offering a multifaceted perspective on its impact across various physiological parameters and clinical outcomes. Our findings agree with previous literature supporting the use of β-blockers in septic shock management. Studies have consistently demonstrated benefits in hemodynamics,32,33 fluid requirements,12,33 and mortality rates associated with β-blocker therapy.34–36 For instance, Morelli et al.1 found that esmolol significantly improved hemodynamic stability and reduced mortality in patients with septic shock. Similarly, our findings support the outcomes observed in the study by Fuchs et al.,26 which explored the continuation of chronic beta-blocker therapy in patients with septic shock, highlighting potential benefits in maintaining beta-blocker therapy during acute illness, including improvements in hemodynamic stability and possibly mortality outcomes. Another study by Macchia et al.34 demonstrated a significant reduction in mortality rates with β-blocker use in septic patients.
LIMITATIONS
The study has some limitations. The sample size of patients may not be adequate to generalize the findings to the broader population of patients. The single-center design restricts the applicability to different clinical settings. Measurement timing may have missed dynamic changes, affecting accuracy. Excluding patients with adverse events may underestimate risks. These limitations highlight the need for larger, multi-center trials and comprehensive follow-up.
CONCLUSION
In conclusion, our results suggest that the administration of metoprolol in septic shock secondary to VAP significantly improves hemodynamics, enhances tissue perfusion, and contributes to favourable clinical outcomes. The observed modulation of heart rate dynamics and the associated anti-inflammatory effects underscore the potential therapeutic benefits of metoprolol in the intricate landscape of septic shock management. Further exploration through larger-scale trials is warranted to validate and refine our observations and perhaps identify precision interventions in critical care.
ACKNOWLEDGMENTS
The authors express their heartfelt appreciation to their colleagues, whose collaboration has been integral to the success of this research. Finally, deep gratitude is given to the author’s family for their continuous support. This accomplishment would not have been possible without their unwavering presence and understanding.
DATA AVAILABILITY STATEMENT
The datasets generated and analyzed during the current study are not publicly available due to privacy and ethical restrictions but are available from the corresponding author on reasonable request. Any shared data will be de-identified to ensure patient confidentiality and compliance with ethical standards.
REFERENCES
- Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of global incidence and mortality of hospital-treated sepsis current estimates and limitations. Am J Respir Crit Care Med 2016;193(3):259–72.
- Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 2021;47(11):1181–247.
- Kalantari A, Mallemat H, Weingart SD. Sepsis definitions: the search for gold and what cms got wrong. West J Emerg Med 2017;18(5):951–6.
- Ehlenbach WJ, Curtis JR. Noninvasive ventilation for patients near the end of life: what do we know and what do we need to know? Crit Care Med 2008;36(3):1003–4.
- van Vught LA, Wiewel MA, Hoogendijk AJ, et al. The host response in patients with sepsis developing intensive care unit-acquired secondary infections. Am J Respir Crit Care Med 2017;196(4):458–70.
- Bridges EJ, Dukes S. Cardiovascular aspects of septic shock: pathophysiology, monitoring, and treatment. Crit Care Nurse. 2005;25(2):14–6, 8–20, 2–4 passim; quiz 41–2.
- Paul M, Shani V, Muchtar E, et al. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrobial Agents and Chemotherapy 2010;54(11):4851–63.
- van der Heijden C, Groh L, Keating ST, et al. Catecholamines induce trained immunity in monocytes In Vitro and In Vivo. Circ Res 2020;127(2):269–83.
- Nambiar V, Chalappurath D. Thrombotic tendencies in excess catecholamine states. Biogenic Amines in Neurotransmission and Human Disease 2019. DOI: 10.5772/intechopen.81929
- Chioléro R, Revelly J-P, Tappy L. Energy metabolism in sepsis and injury. Nutrition 1997;13(9, Supplement 1):45–51.
- Kumar A, Pappachan JM, Fernandez CJ. Catecholamine-induced cardiomyopathy: an endocrinologist’s perspective. Rev Cardiovasc Med 2021;22(4):1215–28.
- Morelli A, Ertmer C, Westphal M, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA 2013;310(16):1683–91.
- Gattinoni L, Brazzi L, Pelosi P, et al. A trial of goal-oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med 1995;333(16):1025–32.
- Parker MM, Shelhamer JH, Natanson C, et al. Serial cardiovascular variables in survivors and nonsurvivors of human septic shock: heart rate as an early predictor of prognosis. Crit Care Med 1987;15(10):923–9.
- Morris J, Awosika AO, Dunham A. Metoprolol. StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC.; 2024.
- Ginsberg F. β-blockers: more good news? Crit Care Med 2012;40(10):2901–2.
- Vimala LR, Eifer DA, Karimzad Y, et al. Prospective clinical trial comparing iv esmolol to iv metoprolol in ct coronary angiography: effect on hemodynamic, technical parameters and cost. Can Assoc Radiol J. 2022;73(1):240–8.
- Novotny NM, Lahm T, Markel TA, et al. Beta-blockers in sepsis: reexamining the evidence. Shock 2009;31(2):113–9.
- Vieillard-Baron A, Caille V, Charron C, et al. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med 2008;36(6):1701–6.
- Yang YL, Xiang ZJ, Yang JH, et al. Association of β-blocker use with survival and pulmonary function in patients with chronic obstructive pulmonary and cardiovascular disease: a systematic review and meta-analysis. Eur Heart J 2020;41(46):4415–22.
- Alberti KG, Batstone GF, Foster KJ, et al. Relative role of various hormones in mediating the metabolic response to injury. JPEN J Parenter Enteral Nutr 1980;4(2):141–6.
- Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clinical Infectious Diseases 2016;63(5):e61–e111.
- Magill SS, Klompas M, Balk R, et al. Developing a new, national approach to surveillance for ventilator-associated events. Am J Crit Care 2013;22(6):469–73.
- Spalding MC, Cripps MW, Minshall CT. Ventilator-associated pneumonia: new definitions. Crit Care Clin. 2017;33(2):277–92.
- Ramos Corrêa Pinto L, Azzolin KO, Lucena AF, et al. Septic shock: Clinical indicators and implications to critical patient care. J Clin Nurs 2021;30(11–12):1607–14.
- Fuchs C, Wauschkuhn S, Scheer C, et al. Continuing chronic beta-blockade in the acute phase of severe sepsis and septic shock is associated with decreased mortality rates up to 90 days. Br J Anaesth 2017;119(4):616–25.
- Singer KE, Collins CE, Flahive JM, et al. Outpatient beta-blockers and survival from sepsis: Results from a national cohort of Medicare beneficiaries. Am J Surg 2017;214(4):577–82.
- Morelli A, Passariello M. Hemodynamic coherence in sepsis. Best Pract Res Clin Anaesthesiol 2016;30(4):453–63.
- Baygin O, Kararmaz A. Sepsis and tachycardia: etiologic factors and effects on prognosis. Ther Anesth J 2018;1:103.
- Carrara M, Ferrario M, Bollen Pinto B, et al. The autonomic nervous system in septic shock and its role as a future therapeutic target: a narrative review. Ann Intensive Care 2021;11(1):80.
- Morelli A, D’Egidio A, Passariello M. Tachycardia in septic shock: pathophysiological implications and pharmacological treatment. In: Vincent J-L, editor. Annual Update in Intensive Care and Emergency Medicine 2015. Cham: Springer International Publishing; 2015. p. 115–28.
- Tao Y, Jingyi W, Xiaogan J, et al. [Effect of esmolol on fluid responsiveness and hemodynamic parameters in patients with septic shock]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2015;27(11):885–9.
- Schmittinger CA, Dünser MW, Haller M, et al. Combined milrinone and enteral metoprolol therapy in patients with septic myocardial depression. Crit Care 2008;12(4):R99.
- Macchia A, Romero M, Comignani PD, et al. Previous prescription of β-blockers is associated with reduced mortality among patients hospitalized in intensive care units for sepsis. Crit Care Med 2012;40(10):2768–72.
- Li J, Sun W, Guo Y, et al. Prognosis of β-adrenergic blockade therapy on septic shock and sepsis: A systematic review and meta-analysis of randomized controlled studies. Cytokine. 2020;126:154916.
- Hasegawa D, Sato R, Prasitlumkum N, et al. Effect of ultrashort-acting β-blockers on mortality in patients with sepsis with persistent tachycardia despite initial resuscitation: a systematic review and meta-analysis of randomized controlled trials. Chest 021;159(6):2289–300.
Article citation: Fayed AM, Meligy HS, Hamad SR. The impact of intravenous metoprolol tartrate on mortality rates in patients with septic shock due to ventilator-associated pneumonia: a randomized clinical trial. The Southwest Respiratory and Critical Care Chronicles 2024;12(53):1–11
From: Alexandria Main University Hospital, Alexandria, Egypt
Submitted: 7/28/2024
Accepted: 9/19/2024
Conflicts of interest: none
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.