INTRODUCTION
Primary adrenal insufficiency, or Addison’s disease, is a disorder of the adrenal gland that results from decreased production of mineralocorticoids and glucocorticoids. Although a relatively rare condition with a prevalence of 93–140 per million and an incidence of 4.7–6.2 per million,1 Addison’s disease can be fatal, particularly in pregnant women, since pregnancy itself is a vulnerable, “stressful” condition that requires special medical attention.
Pregnancy can occur in women with chronic adrenal insufficiency, but acute adrenal crisis can also develop as a new diagnosis during pregnancy. Therefore, presentations vary depending on the time course for the adrenal disorder and can mimic some physiological changes of pregnancy further complicating the initial diagnosis. Some physical findings, e.g., unusually low systolic blood pressure, and symptoms, such as excessive vomiting, severe unexplained fatigue etc., during pregnancy can lead to the suspicion of Addison’s disease. Serum electrolyte measurement followed by more specific serum cortisol, serum adrenocorticotropic hormone (ACTH), and the short synacthen test can help establish the diagnosis. Early diagnosis and definitive treatment with appropriate hormone supplementation can ensure an uneventful pregnancy with good outcomes.
This review briefly summarizes the current knowledge on both the pathophysiology and the clinical aspects of pregnancy in Addison’s disease based on case reports and to make recommendations for the management of pregnant patients with Addison’s disease.
NORMAL ADRENAL RESPONSE IN PREGNANCY
There is increased activity of the hypothalamic-pituitary-adrenal (HPA) axis during pregnancy (Figure 1). Maternal ACTH, cortisol, urinary free cortisol, androgen, plasma renin activity, and aldosterone levels are also increased during pregnancy; but there is no clinical evidence for either hyperaldosteronism or hypercortisolism.2 The clear etiology of increased ACTH is not known but may include synthesis and release of corticotropin releasing hormone (CRH) and ACTH, increased pituitary response to vasopressin and CRH, and desensitization of the pituitary to cortisol feedback. Estrogen produced by the placenta stimulates the liver to produce corticosteroid binding globulin, which causes a reduction of free cortisol level and helps maintain the normal feedback response. Plasma cortisol is increased 2–3-fold during gestation.3 There is a several hundred-fold increase in placental CRH during gestation, which reaches a very high concentration at term.4 During the postpartum period, the HPA axis activity and maternal plasma cortisol levels gradually return to a pre-pregnant state.5
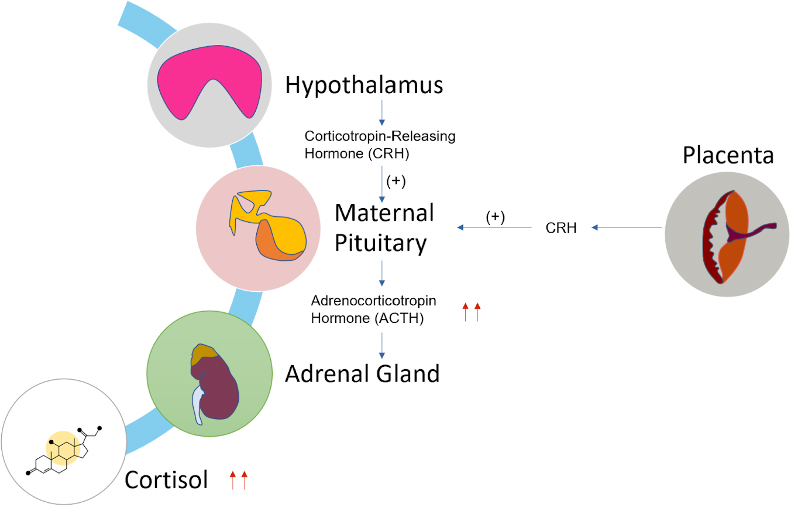
Figure 1. Changes in HPA axis during pregnancy.
During pregnancy, significant changes occur in the renin-angiotensin-aldosterone system (RAAS) (Figure 2). Plasma renin activity is increased early in pregnancy and by the third trimester reaches values 3–7-fold above the normal range. There is a 5–20-fold increase in plasma aldosterone level.6 Plasma cortisol is increased 2–3-fold during gestation.3 There is a several hundred-fold increase in placental CRH during gestation, which reaches a very high concentration at term.4
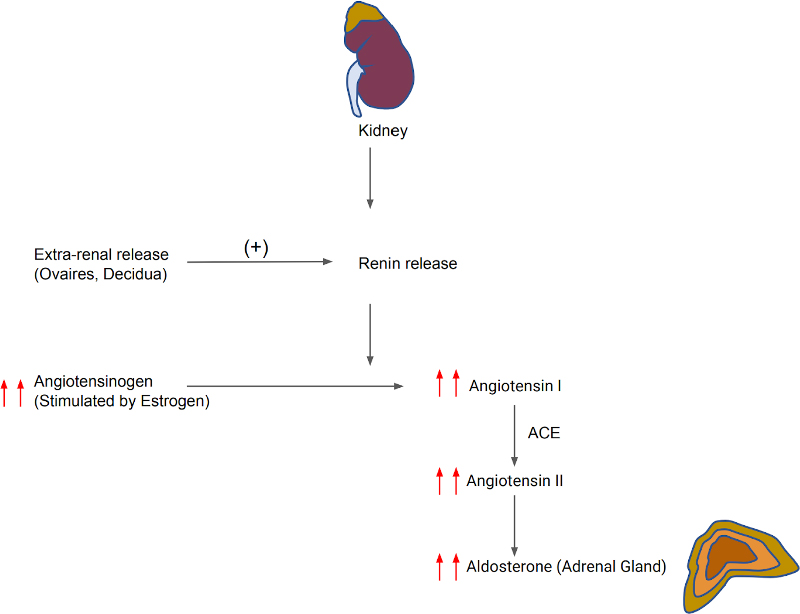
Figure 2. Changes in RAAS system during pregnancy.
Renin release is also increased in both kidney and extrarenal tissues (e.g., ovaries, decidua).7 Plasma volume expansion occurs due to increased aldosterone levels; this effect is reduced by increased progesterone of pregnancy, which acts as an antagonist of mineralocorticoid receptors and reduces sodium reabsorption. Angiotensinogen synthesis is increased in the liver by the stimulation of estrogen; there is also increased circulating levels of angiotensin II. The vasopressor effect of angiotensin II is compensated by several mechanisms, particularly the release of prostaglandin from the uteroplacental unit which reduces systemic vascular resistance and arterial blood pressure.7
ADRENAL INSUFFICIENCY
Adrenal insufficiency results from inadequate secretion of cortisol and/or aldosterone. It is potentially fatal and notoriously variable in its presentation. Therefore, a high index of suspicion is required in patients presenting with unexplained fatigue, hyponatremia, or hypotension. Patients may present with chronic features and/or with acute circulatory shock. With a chronic presentation, initial symptoms are often misdiagnosed as chronic fatigue syndrome or depression. In primary adrenal insufficiency, weight loss is a uniform presenting feature. Adrenocortical insufficiency should also be considered in patients with hyponatremia, even in the absence of symptoms. Features of acute adrenal crises include circulatory shock with severe hypotension, hyponatremia, hyperkalemia, hypoglycemia, and hypercalcemia. Muscle cramps, nausea, vomiting, diarrhea, and unexplained fever may be present. The crisis is often precipitated by intercurrent illness, surgery or infection. Vitiligo occurs in 10%–20% patients with autoimmune Addison’s disease.8
Adrenal insufficiency in pregnancy is relatively rare, but it is associated with significant maternal and fetal morbidity and mortality if untreated during gestation or in the puerperium.9 During pregnancy, the incidence of adrenal insufficiency is still unknown, and literature data have reported values ranging from 5.6 per 100000 and to 9.6 per 100000.10 Some symptoms associated with pregnancy overlap with those of adrenal insufficiency, which causes difficulty in recognition of this condition in pregnancy. Moreover, adrenal insufficiency diagnosis is complicated due to the clinical presentation of abdominal pain, vomiting, and shock. During pregnancy with adrenal insufficiency, this condition can be mistaken for acute abdomen and hyperemesis gravidarum. The diagnosis of adrenal crisis is based on at least two of the following sign or symptoms: hypotension (systolic blood pressure <100 mmHg), nausea, emesis, severe fatigue, hyponatremia, hypoglycemia, and hyperkalemia, which require parenteral glucocorticoid therapy.11 Therefore, Bornstein et al. recommended performing a short cosyntropin test to detect adrenal insufficiency in pregnant women with unexplained persistent nausea, fatigue, and hypotension.12
Pregnancy dramatically affects the HPA axis resulting in increased circulating cortisol and ACTH levels during gestation. The causes of increased ACTH include placental synthesis, pituitary desensitization to cortisol feedback, or enhanced pituitary responses to corticotropin releasing factors. However, a clear-cut cortisol reference range in pregnancy has not been established, and this has created difficulty in diagnosing adrenal insufficiency during pregnancy. Serum cortisol and the cosyntropin stimulation test are currently the diagnostic tests of choice. Therefore, short cosyntropin tests should be used to detect adrenal insufficiency in pregnant women with unexplained persistent nausea, fatigue, and hypotension.
CASE REPORTS ON ADRENAL INSUFFICIENCY IN PREGNANCY
Several databases, including Google, Google Scholar, Medline, and PubMed, were used to search for relevant articles with the keywords (“Pregnancy,” “Addison’s disease,” “Outcome,” etc.). The date range for publication was 1952 through 2023. This search identified 5 cases (Table 1).
Tagetti et al. (2021) reported a case of a 32-year-old woman at 16 + 3 gestational weeks with a twin pregnancy who presented with persistent vomiting with difficulty feeding, weakness, and mild pelvic pain during the 1st trimester of pregnancy. On admission, the patient’s blood pressure was 90/60 mmHg, temperature 35.4°C, serum sodium 117 mmol/L, potassium 4.68 mmol/L, chloride 64 mmol/L, TSH 1.19 mUI/L, FT3 4 pg/mL, FT4 11.5 ng/L, ACTH 648 pg/mL, cortisol 14.5 μg/dL, and plasma renin >500 μU/mL; aldosterone was 65 pg/mL standing and 67 pg/mL supine. Her past history included celiac disease and Hashimoto thyroiditis. The patient was treated with 100 mg of intravenous (IV) hydrocortisone three times a day (tid), then 50 mg IV tid because of hyperglycemia. After five days, oral therapy was started with hydrocortisone 10 mg tid plus fludrocortisone 100 μg which was increased to 200 μg after 6 days. The patient was stable on medications, and her pregnancy was terminated at 35 + 2 weeks with the birth of 2 female babies. The patient was discharged home with hydrocortisone 10 + 5 + 5 mg + fludrocortisone 150 μg combination. During the next 5 months, fludrocortisone was gradually reduced to 50 μg/d for edema and low plasma renin. She had no symptoms afterwards.13
Margulies et al. (2020) reported a case of a 34-year-old previously healthy G2P1001 patient who presented with lethargy, skin hyperpigmentation, polyuria, and salt craving. Laboratory findings were significant with hyperkalemia, hyponatremia, elevated ACTH, and low cortisol. The pregnancy was terminated early; she was placed on a regimen of hydrocortisone and fludrocortisone, leading to symptom resolution. On her second presentation as a G5P1031, she was started on hydrocortisone and fludrocortisone regimen. She had recurrent symptoms secondary to adrenal insufficiency, and her ACTH levels were checked to determine if her current medications could be optimized. She delivered a healthy male vaginally. For her third presentation as a G6P2032, her pregnancy was managed similarly to the previous pregnancy with a successful pregnancy ending with healthy birth.14
Mittal et al. (2011) reported a case of a 34-year-old pregnant female in her third pregnancy at 16 weeks of gestation, who presented with loss of appetite, extreme exhaustion, muscle and joint aches, headache, and solid food sticking in her throat. She also had mild nausea and salt-craving behavior. The patient mentioned struggling to walk for 5 min on the flat, had to crawl upstairs, and had to rest after the slightest activity. She felt faint most of the time when upright and relied on physical support from her husband, lost 10 kg of weight in 4 months, and noticed brown staining to her lips and gums. Examination revealed a cachectic appearance with brown pigmentation to the lower lips, gums, and palmar creases. Her pulse was 70 beats per minute and regular, and blood pressure was 80/55 mmHg in the supine position. Serum sodium was low, ACTH was grossly elevated, and cortisol was low. The short synacthen test was negative. The presence of adrenal antibodies was confirmed as primary adrenal insufficiency. Treatment was started with hydrocortisone 20 mg in the morning and 10 mg in the evening, with fludrocortisone 0.1 mg daily added on day four. Serum electrolytes became normal, and her blood pressure (BP) stabilized. A Cesarean delivery was done, and her post-delivery course was uneventful. To reduce the risk of precipitating an Addisonian crisis, she received oral steroids as premedication, in addition to her usual medication doses; hydrocortisone 15 mg and fludrocortisone 0.1 mg. Intraoperatively, she received 100 mg IV hydrocortisone and then 50 mg IV hydrocortisone every 6 hours for the initial 24 hours post-delivery.15
Lewandowski et al. (2010) reported a case of a 32-year-old woman in her second pregnancy at 14 weeks of gestation who presented with hypotension and weight loss. This patient had a past history of primary hypothyroidism treated with thyroxine (100 microgram/ day). She had one prior female child born small for gestational age. At 8 weeks of her second gestation, she started to vomit several times a day and was hospitalized and treated with antiemetics and intravenous fluids. Following discharge, she remained nauseated, weak, and lightheaded and lost about 8 kg of weight. On readmission, she appeared ill and dehydrated; her BMI was 16.6 kg/m2 and her BP was 90/60 mmHg supine and 70/50 mmHg upright (with faint-like sensation). She was hyperpigmented, hypotensive, and hyponatremic despite rehydration. Laboratory tests included sodium 112 mmol/L, potassium level 4.3 mmol/L, normal renal function, TSH 1.31 microIU/mL, freeT4 1.99 ng/dL, freeT3 3.29 pg/mL, anti-TPO antibodies 467 IU/mL. Cortisol and ACTH were measured followed by a 250 microgram short Synacthen test that revealed a peak cortisol response of 17 nmol/L and a high baseline ACTH 969 pg/mL. The diagnosis of Addison’s disease in combination with hypothyroidism resulted in the diagnosis of Autoimmune Polyglandular Syndrome type II. She was discharged on hydrocortisone and fludrocortisone replacement with an excellent response and delivered a healthy female infant at 36 weeks of gestation.16
Richards (1952) reported a case of 35-year-old primigravida who was diagnosed with Addison’s disease previously and was on maintenance dose of fludrocortisone with a 200 mg implant being inserted every 3–6 months based on laboratory values for about 4 years. Patient was admitted to the hospital with severe vomiting with tiredness and fatigue with blood pressure at 70/40 mmHg and was found to be pregnant. Laboratory values were significant with urinary sodium chloride to be 14 gm/L (the Fantus method used for testing). She was treated with intravenous saline and salt intake was increased to 15 gm daily and Eucortone (an extract of adrenal cortex) 20 mL daily for 3 days. Despite this regimen, she was still feeling fatigued and had persistent vomiting. She was given 5 mg fludrocortisone twice a week. She had vaginal bleeding and was treated as a threatened abortion with bed rest with maintenance dose of fludrocortisone 10 mg with reduction of salt intake. Her BP stabilized. She was hospitalized again with abdominal pain and was found to have a twin pregnancy instead of a singleton. She had a spontaneous delivery of male twins prematurely at 34th week. She was treated with 5% dextrose normal saline drip with 2–3 L given daily for 4 days with continued use of fludrocortisone 10 mg daily with supplementary Eucortone. She stabilized afterwards with normalization of her BP. The dosage of fludrocortisone and Eucortone were subsequently reduced. Later she had 3 episodes of hypoglycemia with exertional faintness; however, after those 3 episodes, she did not have additional episodes afterwards and her oral glucose tolerance test was normal.17
DISCUSSION
Addison’s disease in pregnancy is relatively rare, but due to an increase in autoimmune disease, the incidence of Addison’s disease is increasing.2 It is also an under-reported disease as presentations mimic many early symptoms of pregnancy. As a result, patients may consult different physicians without significant improvement, and they ultimately present to a tertiary care center.15 A high index of suspicion is supported by a personal/ family history of autoimmune disease.
The clinical features of Addison’s disease in pregnancy are the same as in non-pregnant patients. Patients often present with nonspecific symptoms, such as fatigue, lethargy, anorexia, nausea, gastric pain, dizziness, etc. Due to their resemblance with normal symptoms of pregnancy, e.g., vomiting in hyperemesis gravidarum, these symptoms can be misleading; a more severe presentation that persists beyond the first trimester and does not resolve with usual treatment approaches should call for a more extensive evaluation.
ACTH-driven Addisonian hyperpigmentation is a more specific sign of primary adrenal insufficiency and is distributed along areas of increased mechanical frictions, such as scars, nipples, knuckles, toes, and even the oral mucosa in contact with teeth. Postural hypotension is a common presentation in chronic mineralocorticoid deficiency; severe hypotension or even hypovolemic shock with acute abdominal pain, vomiting, and often fever suggests acute adrenal insufficiency or Addisonian crisis.7 These crises may occur more frequently during early pregnancy secondary to illness or during the peripartum period secondary to labor or delivery.
Based on a strong clinical suspicion, the first tests should include electrolytes that will reveal hyponatremia (or a reduction in serum sodium >5 mmol/L) and hyperkalemia and a random blood sugar to check for hypoglycemia and complete blood counts to check for eosinophilia and lymphocytosis. Serum potassium may be normal due to pregnancy-induced increase in RAAS.4 These results should prompt measurements of paired plasma cortisol and ACTH levels at baseline, followed by a short synacthen test with injection of 250 mcg of ACTH.7 Plasma ACTH level >22 pmol/L with random morning cortisol levels of <300, <450 and <600 nmol/L increase the suspicion for adrenal insufficiency when measured during the first, second, and third trimester of pregnancy, respectively, as total cortisol levels increase progressively throughout pregnancy with a concurrent rise of capillary blood glucose.7 Baseline and stimulated salivary cortisol concentration can also be used as a surrogate marker for establishing the diagnosis.4
The short synacthen test is the most convenient test providing a valid measure of adrenal cortisol reserve. Cut-off values in these tests are 700, 800, and 900 nmol/L for the first, second and third trimester of pregnancy, respectively; failure to reach these levels would establish the diagnosis of adrenal insufficiency in the context of pregnancy. Irrespective of the results, it is important to start a therapeutic trial with intravenous (IV) hydrocortisone in the case of adrenal crisis to avert significant morbidity and mortality associated with delay in initiating treatment.7
Adequate replacement of adrenal hormones virtually guarantees an uneventful pregnancy with acute adrenal insufficiency.2 The aim is to achieve a physiological glucocorticoid replacement dose. Among the different glucocorticoids available, hydrocortisone is the drug of choice as it is more physiologic, and it does not cross the placenta due to degradation by 11b-hydroxysteroid dehydrogenase type 2.2 The recommended dose is 12 to 15 mg/m2 of body surface area, and it should be adjusted according to clinical judgment with a 20–40% increased dosage in the third trimester. Doses should be increased at times of stress and in the peripartum period. Generally, hydrocortisone 50 mg is given IV in the second stage of labor and further dosing needs to be adjusted according to the course of labor. In the case of cesarean delivery, initially, 100 mg hydrocortisone should be given IV or IM, and then every 6 to 8 hours, with tapering the dose in the next 48 hours.4 Since progesterone has anti-mineralocorticoid effect, fludrocortisone replacement in primary adrenal insufficiency is started at doses of 0.1 mg (with a range varying from 0.05 to 0.25 mg). Although the concentration of progesterone increases steadily throughout pregnancy, its increasing anti-mineralocorticoid effect is mitigated by administering increasing doses of hydrocortisone, as hydrocortisone also exerts some activity on mineralocorticoid receptors. Measurement of plasma renin is not reliable for monitoring mineralocorticoid replacement as renin secretion increases normally during pregnancy. Rather regular measurements of blood pressure (supine/erect) and serum electrolytes are more feasible.7 Patients with a history of receiving glucocorticoid as anti-inflammatory therapy within 1 year should be treated with stress doses of glucocorticoids during labor and delivery due to adrenal axis suppression.4
CONCLUSION
Adrenal disorders in pregnancy are uncommon but can be associated with considerable fetal and maternal mortality and morbidity if not recognized and treated. The key problem is the early identification of a pregnant woman in whom Addison’s disease recently appeared, because symptoms and signs are often ambiguous and resemble the clinical picture of a normal pregnancy. With a correct diagnosis and if the treatment is adequate, pregnancy, labor, and delivery can occur uneventfully. Therefore, pregnancy and Addison’s disease are not incompatible, as long as proper management is provided.
REFERENCES
- Kenward D, White KG. Adrenal insufficiency. The Lancet 2003;362(9383):579–80. doi: https://doi.org/10.1016/S0140-6736(03)14133-5.
- Ambrosi B, Barbetta L, Morricone L. Diagnosis and management of Addison’s disease during pregnancy. J Endocrinol Invest 2003;26(7):698–702. doi: 10.1007/BF03347034.
- Lindsay JR, Nieman LK. The hypothalamic-pituitary-adrenal axis in pregnancy: challenges in disease detection and treatment. Endocr Rev 2005;26(6):775–99. doi:10.1210/er.2004-0025.
- Kamoun M, Mnif MF, Charfi N, et al. Adrenal diseases during pregnancy: pathophysiology, diagnosis and management strategies. Am J Med Sci 2014;347(1):64–73. doi: 10.1097/MAJ.0b013e31828aaeee.
- Duthie L, Reynolds RM. Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: influences on maternal and fetal outcomes. Neuroendocrinology 2013;98(2):106–15. doi: 10.1159/000354702.
- Lekarev O, New MI. Adrenal disease in pregnancy. Best Pract Res Clin Endocrinol Metab 2011;25(6):959–73. doi: 10.1016/j.beem.2011.08.004.
- Lebbe M, Arlt W. What is the best diagnostic and therapeutic management strategy for an Addison patient during pregnancy? Clin Endocrinol (Oxf) 2013;78(4):497–502. doi: 10.1111/cen.12097.
- Newell-Price JDC, Gibb FW. Endocrinology, Davidson’s Principles and Practice of Medicine, 24th ed. 2023.
- Yuen KC, Chong LE, Koch CA. Adrenal insufficiency in pregnancy: challenging issues in diagnosis and management. Endocrine 2013;44(2):283–92. doi: 10.1007/s12020-013-9893-2.
- Schneiderman M, Czuzoj-Shulman N, Spence AR, et al. Maternal and neonatal outcomes of pregnancies in women with Addison’s disease: a population-based cohort study on 7.7 million births. BJOG 2017;124(11):1772–9. doi: 10.1111/1471-0528.14448.
- Allolio B. Extensive expertise in endocrinology. Adrenal crisis. Eur J Endocrinol 2015;172(3):R115–24. doi: 10.1530/EJE-14-0824.
- Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2016;101(2):364–89. doi: 10.1210/jc.2015-1710.
- Tagetti A, Marcon D, Moghetti P, et al. Onset of Addison Disease appeared during the first trimester of a twin pregnancy: A case report. Clin Case Rep 2021;9(5):e03784. doi: 10.1002/ccr3.3784.
- Margulies SL, Corrigan K, Bathgate S, et al. Addison’s disease in pregnancy: Case report, management, and review of the literature. J Neonatal Perinatal Med 2020;13(2):275–8. doi: 10.3233/NPM-190231.
- Mittal A, Dexter S, Marcus S, et al. First presentation of Addison’s disease in the 2nd trimester of pregnancy. J Obstet Gynaecol 2011;31(4):342. doi: 10.3109/01443615.2011.563330.
- Lewandowski K, Hincz P, Grzesiak M, et al. New onset Addison’s disease presenting as prolonged hyperemesis in early pregnancy. Ginekol Pol. 2010;81(7):53–40.
- Richards TA. Addison’s disease and pregnancy. Br Med J. 1952;1(4755):421. doi: 10.1136/bmj.1.4755.421.
Article citation: Hasan SM, Sharmin FH, Haque S, Afrin S, Paul SP, Al Masud A, Rimu AH. Addison’s disease in pregnancy. The Southwest Respiratory and Critical Care Chronicles 2025;13(54):9–16
From: Department of Medicine (SHH), Dhaka Medical College Hospital, Dhaka, Bangladesh; Khulna Medical College Hospital (FHS), Khulna, Bangladesh; Department of Internal Medicine (SH), Jersey Shore University Medical Center, Neptune, NJ, USA; Department of Internal Medicine (SA), St. Elizabeth Youngstown Hospital, Youngstown, Ohio, USA; Dept of Rheumatology (SPP), Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh; St Louis University School of Medicine (AAM), St Louis, MO; Department of Internal Medicine (AHR), Texas Tech University Health Sciences Center, Lubbock, Texas, USA.
Submitted: 9/23/2024
Accepted: 12/24/2024
Conflicts of interest: none
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.