Abstract
Average volume-assured pressure support versus fixed pressure support in chronic hypercapnic respiratory failure: A systematic review and meta-analysis
Abbie Evans MD, Aayan Alam, Kenneth Nugent MD
Corresponding author: Abbie Evans
Contact Information: Abbie.Evans@ttuhsc.edu
DOI: 10.12746/swrccc.v12i53.1383
ABSTRACT
Rationale: Chronic hypercapnic respiratory failure occurs due to alveolar hypoventilation resulting in carbon dioxide retention. This is commonly managed with noninvasive ventilation (NIV) with modalities including fixed pressure support and average volume-assured pressure support (AVAPS). However, there is limited information comparing outcomes with these two modes of ventilator support in the management of chronic hypercapnic respiratory failure.
Objective: This review and meta-analysis analyze the outcomes with fixed pressure NIV versus average volume-assured pressure support NIV in managing chronic obstructive pulmonary disease (COPD) with chronic hypercapnic respiratory failure, focusing on patients’ perception of symptom burden and gas exchange based on arterial blood gases.
Search methods: PubMed, Embase, Cochrane Central Register of Controlled Trials, and Web of Science databases were searched; the latest search date was December 1, 2023. Inclusion criteria: randomized control trials and crossover studies in English in adults over the age of 19 with the diagnosis of COPD and chronic hypercapnic respiratory failure. Exclusion criteria: patients less than 19 years old, patients with acute exacerbations, and patients with central respiratory failure or neuromuscular disease. Outcomes included blood gas analysis after use of NIV measured in mmHg and patient perception of mental health, symptom burden, and comfort. Results for each outcome were analyzed in RevMan using an inverse variance statistical method with a fixed effect analysis. The final analysis included 7 studies with 252 participants.
Results: The patients were 64 ± 9 years old. Baseline pulmonary function testing showed a forced expiratory volume in the first second (FEV1) of 34.6 ± 14.2% predicted, consistent with severe COPD per GOLD criteria, and a baseline PaCO2 of 55.2 ± 9.2 mmHg. Primary outcomes for ventilation showed no statistical difference between AVAPS and fixed pressure support groups in PaCO2 (Odds Ratio [OR] −1.51; 95% Confidence Interval [CI]: −3.18, 0.16; p = 0.08). Patient perceived outcomes were evaluated using several questionnaires, including St. George Respiratory Questionnaire (SGRQ), Short Form 36 Health Survey Questionnaire (SF-36), and Visual Analogue Scale (VAS). Comparable results were not available for all studies, but no statistically significant differences were found when comparing study results.
Conclusions: There was little or no clinically significant difference between fixed pressure support and AVAPS in gas exchange. There are inadequate data to draw conclusions about the effect of fixed pressure support compared to AVAPS on patient perceived outcomes, such as comfort and symptom burden. No studies evaluated mortality benefit, cost effectiveness, or hospitalizations.
Keywords: COPD, chronic hypercapnic respiratory failure, non-invasive ventilation, average volume-assured pressure support ventilation
INTRODUCTION
Chronic hypercapnic respiratory failure occurs due to alveolar hypoventilation secondary to several conditions, including mechanical dysfunction of the respiratory system and an imbalance between carbon dioxide production and elimination. It ultimately results in carbon dioxide retention and is commonly managed with noninvasive ventilation (NIV).1 Two commonly used NIV modalities are fixed pressure support and average volume-assured pressure support (AVAPS), which integrates volume and pressure control by using targeted tidal volumes with dynamic inspiratory positive airway pressure changes during each breath cycle.2 This review assesses the outcomes with these two noninvasive support strategies in managing chronic hypercapnic respiratory failure in patients with COPD based on recent clinical trials, focusing on patient symptoms and arterial blood gases.
BACKGROUND
Average volume-assured pressure support (AVAPS) combines pressure support with a guaranteed minimum tidal volume. It adapts the pressure provided based on the patient’s inspiratory needs to ensure a consistent tidal volume, potentially improving carbon dioxide clearance. Since its development in 2009, it has been used in chronic respiratory failure secondary to chronic obstructive pulmonary disease (COPD), obesity-hypoventilation syndrome, kyphoscoliosis, and congenital central hypoventilation syndrome. This mode does not use a fixed inspiratory positive airway pressure (IPAP), as in fixed pressure support. Rather, the set parameters include target tidal volume, respiratory rate, expiratory positive airway pressure (EPAP), and a minimum and maximum IPAP. This IPAP range allows the machine to increase or decrease the inspiratory pressure with each breath to ensure adequate volume is delivered, which is thought to improve patient comfort.2
Fixed pressure support, such as bilevel positive airway pressure (BiPAP), provides a constant level of pressure support throughout the respiratory cycle, which may not adjust to changes in the patient’s respiratory mechanics or tidal volume needs.3 Potential benefits of AVAPS includes a more consistent minute ventilation, which theoretically may lead to more stable gas exchange. Complications of AVAPS include financial barriers, mucosal dryness/irritation, claustrophobia, air leaks, facial abrasions secondary to masks, treatment failure, pneumonia, and, rarely, barotrauma.2
Several studies have compared the clinical outcomes of AVAPS and fixed pressure support in patients with chronic hypercapnia with conflicting results. Magdy et al. (2021) found that AVAPS significantly reduced daytime PaCO2 levels on arterial blood gases as compared to fixed pressure support. The authors noted that the adaptive nature of AVAPS led to higher mean tidal volumes and significantly lower air leaks in the AVAPS arm.4 Other studies have reported no significant difference between gas exchange and ventilation between AVAPS and fixed pressure support, suggesting at least non-inferiority of AVAPS compared to standard fixed pressure support.5,6 In addition, AVAPS has been associated with better patient comfort and adherence compared to fixed pressure support. Canpolat et al. (2019) reported that patients on AVAPS reported fewer episodes of discomfort and better tolerance, which may contribute to improved compliance with therapy.7 Other studies have also reported increased patient comfort and improvement in gas exchange based on pH and PaCO2 with AVAPS use; however, these studies had small sample sizes.8
The purpose of this study is to perform a systematic review and meta-analysis of the outcomes with AVAPS as compared to fixed pressure positive pressure ventilation, such as BiPAP, in patients with COPD and hypercapnic respiratory failure. Primary outcomes included arterial blood gases, symptom burden, physical function, and mental health.
METHODS
STUDY IDENTIFICATION
A comprehensive literature search was conducted to identify randomized control trials comparing AVAPS to pressure controlled non-invasive ventilation (NIV). Databases searched included PubMed, Embase, Cochrane Central Register of Controlled Trials, and Web of Science through December 1, 2023. The filters “Randomized Control Trial” and “Age 19+” were used. The search term included “volume-assured pressure support”. A total of 183 articles were identified. Details of article identification and exclusion criteria are displayed in the flow diagram (Figure 1). Of these articles, 7 compared AVAPS to pressure-controlled NIV in patients with COPD and chronic hypercapnic respiratory failure.4,5,9–13
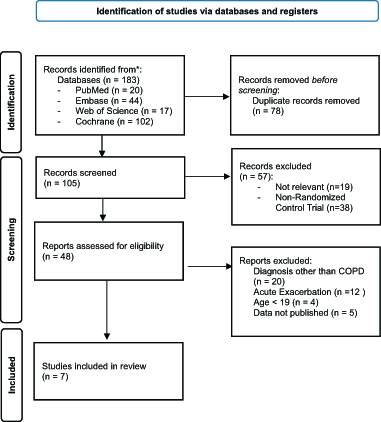
Figure 1. Flow Diagram of Identification and Exclusion of Studies.
From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71
INCLUSION AND EXCLUSION CRITERIA
Inclusion criteria included randomized control trial or crossover study involving the use of AVAPS and fixed pressure support NIV for compensated, chronic respiratory failure in stable COPD adults over the age of 19. The diagnosis of COPD was required to limit data from multiple disease processes confounding results. Finally, only articles in English were included. Exclusion criteria included patients less than 19 years old, patients with acute exacerbations, and patients with central respiratory failure or neuromuscular disease.
OUTCOME MEASURES
Primary outcomes assessed included arterial blood gases after use of NIV and patient symptoms, physical function, and mental health.
DATA COLLECTION
Data were extracted from published studies by two independent reviewers (AE and AA) on the same date and subsequently compared for differences. Data were extracted into RevMan.14
QUALITY ASSESSMENT AND SYNTHESIS METHODS
Studies were assessed for risk of bias in each article using Jadad scale, in which acceptable studies include a score of 3 and above with the highest quality score being 5.15 This scale accounts for study blinding, randomization, and participant withdrawals. Table 1 includes a summary of bias risk for included studies.
Table 1. Characteristics of Included Studies
| Characteristics of Included Studies |
| |
Sample Size |
|
| Study |
Condition |
Setting |
Control |
Treatment |
Design |
Duration |
Primary Outcomes |
| Nilius12 |
CHRF and COPD |
Outpatient |
14 |
14 |
Crossover study |
1 night in each arm |
Respiratory event rate and sleep quality |
| Magdy4 |
CHRF and COPD |
Outpatient |
25 |
25 |
Randomized 1:1 |
5 days |
Treatment efficacy and patient satisfaction |
| Magdy11 |
CHRF and COPD |
Outpatient |
20 |
20 |
Randomized 1:1 |
6 months |
Health-related Quality of Life and Exercise tolerance |
| Oscroft5 |
CHRF and COPD |
Outpatient |
25 |
25 |
Crossover study |
8 weeks in each arm |
Daytime ABG and mean nocturnal O2 saturation |
| Oscroft13 |
CHRF and COPD |
Outpatient |
20 |
20 |
Randomized 1:1 |
3 months |
ABG, mean overnight oximetry, compliance |
| Ekkernkamp10 |
CHRF and COPD |
Outpatient |
14 |
14 |
Crossover study |
6 weeks in each arm |
ABG, VAS score, compliance |
| Crisafulli9 |
CHRF and COPD |
Outpatient |
9 |
9 |
Crossover study |
5 nights in each arm |
ABG, VAS score, sleep efficiency, compliance |
STATISTICAL ANALYSIS
Statistical analysis was performed to examine the standardized weighted mean and 95% confidence intervals (CI) of the baseline characteristics of these studies, including age, body mass index (BMI), results of pulmonary function tests, and blood gas analyses. Results were synthesized when possible, using an inverse variance statistical method with a fixed effect analysis. Statistical heterogeneity was assessed using an I2 value. Studies with I2 less than 50% were considered to have little statistical heterogeneity and were used to estimate the overall summary effect size. This analysis used RevMan (Web Version), and a p value less than 0.05 was considered significant.
RESULTS
SEARCH RESULTS AND STUDY CHARACTERISTICS
This search identified seven RCTs and cross over studies that met inclusion criteria.4,5,9-13 Articles were all in English and were published from 2009 through 2021. Study duration ranged from one night to six months. Study characteristics are summarized in Table 2.
Table 2. Jadad Score of Included Studies
| Jadad Score |
| Study |
Randomization |
Blinding |
Withdrawal and Drop Out |
Jadad Score |
Quality |
| Nilius12 |
2 |
0 |
1 |
3 |
High |
| Magdy4 |
2 |
2 |
1 |
5 |
High |
| Magdy11 |
2 |
0 |
1 |
3 |
High |
| Oscroft5 |
2 |
2 |
1 |
5 |
High |
| Oscroft13 |
2 |
0 |
1 |
3 |
High |
| Ekkernkamp10 |
2 |
0 |
1 |
3 |
High |
| Crisafulli9 |
2 |
2 |
1 |
5 |
High |
OUTCOME RESULTS
The primary outcomes for ventilatory support based on blood gas analysis were available in all seven studies. A total of 252 participants were available for comparison with 126 in the AVAPS group and 126 in the fixed pressure support group. Participants had a mean age of 64 ± 9 years and a body mass index of 29.01 ± 8.22 kg/m2. Baseline pulmonary function testing showed an average forced expiratory volume in the first second (FEV1) of 34.6 ± 14.2% predicted, consistent with severe COPD per GOLD criteria,16 and a baseline PaCO2 of 55.2 ± 9.2 mmHg. Additional results are reported in Table 3. Significant effects on gas exchange were variable in the studies. No statistically significant difference was observed between AVAPS and fixed pressure support groups when comparing post-treatment pH (OR 0.01; 95% CI: −0.0, 0.02; p = 0.06), PaCO2 (OR 1.51; 95% CI: −3.18, 0.16; p =0.08), and PaO2 (OR 1.03; 95% CI −0.51, 2.57; p = 0.19) (Figures 2–4).
Table 3. Baseline Characteristics of Participants in Meta-Analysis
| Mean Averages with Standard Deviations of Baseline Characteristics |
|
Number of Participants |
Mean Average |
Standard Deviation |
| Age (years) |
252 |
64 |
9 |
| BMI (kg/m2) |
252 |
29.01 |
8.22 |
| FEV1 (L) |
252 |
0.74 |
0.32 |
| FEV1 (% predicted) |
212 |
34.61 |
14.2 |
| PaCO2 (mmHg) |
252 |
55.2 |
9.18 |
| PaO2 (mmHg) |
252 |
61.9 |
12.48 |
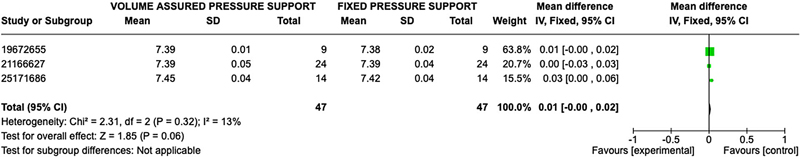
Figure 2. Forest Plot of Mean Difference with Confidence Interval for Post-Treatment pH.
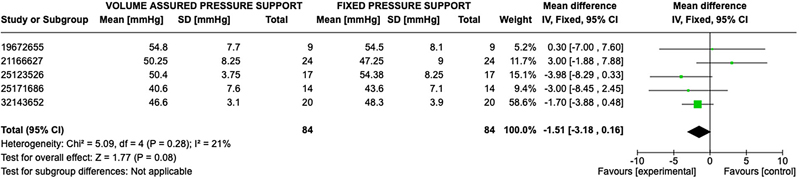
Figure 3. Forest Plot of Mean Difference with Confidence Interval for Post-Treatment PaCO2.
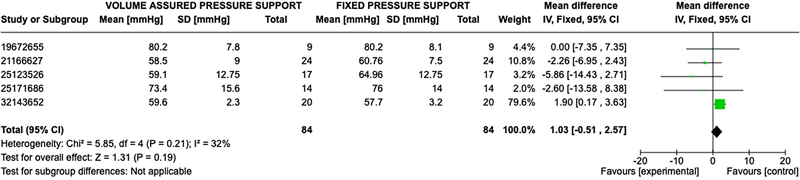
Figure 4. Forest Plot of Mean Difference with Confidence Interval for Post-Treatment PaO2.
Patient perceived outcomes were evaluated through various questionnaires, such as St. George Respiratory Questionnaire (SGRQ), Short Form 36 Health Survey Questionnaire (SF-36), and Visual Analogue Scale (VAS), in each study; therefore, comparable results were not available for all studies. Three studies assessed patients’ posttreatment reported mental health and physical function with the Short Form 36 Questionnaire. Mental health and Physical Function were assessed in 60 patients in the AVAPS group and 60 patients in the fixed pressure support group. No statistically significant difference was observed between AVAPS and fixed pressure support groups when comparing mental health response (OR 0.84; 95% CI: −5.34,7.01; p = 0.79) or physical function response (OR 1.85; 95% CI: −6.02, 9.72; p = 0.64) (Figures 5–6). Two studies assessed patients’ post-treatment reported perception of well-being with the St. George Respiratory Questionnaire with 40 patients in the AVAPS group and 40 patients in fixed pressure support group. No statistical difference was observed between AVAPS and fixed pressure support groups (OR −1.49; 95% CI: −8.57, 5.60; p = 0.68). Four studies assessed patients, post treatment comfort level on a 100-scale visual analog score (100 being most comfort) with 72 patients in the AVAPS group and 72 patients in the fixed pressure support groups. No statistically significant difference was found between AVAPS and fixed pressure support groups (OR −0.01; CI: −4.86, 4.84; p = 1.00). Additional outcomes are reported in the supplementary file.
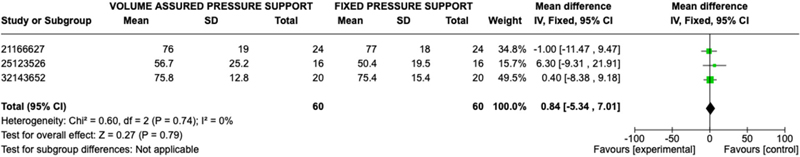
Figure 5. Forest Plot of Mean Difference with Confidence Interval for Post-Treatment SF-36 Mental Health Results.
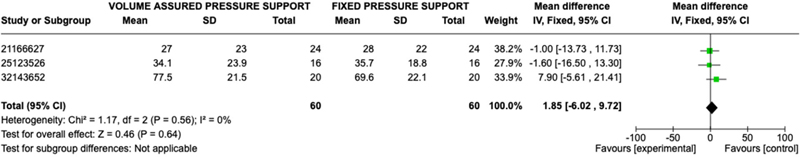
Figure 6. Forest Plot of Mean Difference with Confidence Interval for Post-Treatment SF-36 Physical Function Results.
DISCUSSION
The results in this systematic review and meta-analysis suggest that AVAPS has similar benefits in comparison to fixed pressure support in managing chronic hypercapnic respiratory failure in patients with COPD. Multiple studies included in this review highlighted the potential advantages of AVAPS regarding patient comfort and consequently improved adherence. This is a critical factor in the success of NIV therapies. Patients who experience discomfort may be less likely to use their devices consistently, leading to poorer outcomes. These data suggest that AVAPS users report less discomfort and improved tolerance. This could lead to improved compliance and better clinical outcomes in some patients, if valid, but these differences were not statistically significant.
Despite the overall non-inferiority of AVAPS to fixed pressure support, variability in the results across studies raises important considerations. Factors such as study design, sample size, and duration of therapy likely contribute to the differences in studies. For instance, short term studies may not capture the stability or long-term benefits of AVAPS or fixed pressure support adequately. Furthermore, variations in patient characteristics, such as severity of COPD and baseline respiratory function, could affect outcomes. The differences in studies and in patient cohorts highlight the need for more extensive, multi-center trials that can control for these variables and provide more robust conclusions.
Blood gas analysis serves as a critical objective measure of ventilatory support in evaluating NIV modalities. The results indicate that patient is on both AVAPS and fixed pressure had similar blood gas parameters, particularly PaCO2, with an overall effect likely favoring AVAPS (mean difference 1.03; 95% CI: −0.51, 2.57) but without statistical significance. It is essential to consider how these changes in blood gas translate to clinical outcomes. While improvement in blood gases seems important, it is unclear if the small differences are associated with better quality of life or functional status of patients with COPD and chronic respiratory failure. Additional studies should aim to correlate blood gas improvements with clinically meaningful outcomes, such as exercise tolerance, symptoms relief, and hospitalization.
LIMITATIONS
Several limitations were noted in the included studies. These include small sample sizes based only on published data, limited available data across all studies, multiple methods of data collection, and variability in measurement techniques for patient-reported outcomes. While the blood gas analysis is more objective and standardized, these were collected in two different measurements, both kilopascals and millimeters of mercury. Data were converted to millimeters of mercury for standardization, but this might introduce error. In addition, studies were conducted over various time frames, from as short as 1 night up to 6 months. They large differences in study duration could introduce differences and outcome. Standardizing assessment methods and studying larger, more diverse populations at various disease states could enhance the reliability of findings. Moreover, the absence of long-term data on mortality, hospitalization rates, and cost-effectiveness further complicates the ability to draw a definitive conclusion about the superiority of one modality over the other. Long-term studies focusing on outcomes beyond immediate ventilatory support and blood gases, such as quality of life, economic impact, hospitalizations, and mortality, will be crucial for guiding clinical practice and improving patient care.
CONCLUSION
Average Volume-Assured Pressure Support may offer slight advantages in terms of ventilation support based on PaCO2 and in enhanced quality of life and patient comfort in the management of chronic hypercapnic respiratory failure in comparison to fixed pressure support. However, fixed pressure support remains a good option for patients with a stable respiratory status or when simplicity and ease of use are prioritized, and the choice of support should be guided by individual patient needs and clinical contexts. No studies in this review evaluated mortality benefit, cost effectiveness, or hospitalizations.
SUPPLEMENTARY FILES
Figure 7. Forest plot of mean difference with confidence interval for post-treatment St. George Respiratory Questionnaire
Figure 8. Forest plot of mean difference with confidence interval for post-treatment VAS Comfort Scale
REFERENCES
- Roussos C, Koutsoukou A. Respiratory failure. Eur Respir J Suppl Nov 2003;47:3s–14s. doi:10.1183/09031936.03.00038503
- Yarrarapu SNS, Saunders H, Sanghavi DK. Average volume-assured pressure support. StatPearls. StatPearls Publishing. Copyright © 2023.
- Gong Y SA. Noninvasive Ventilation. StatPearls. StatPearls Publishing Copyright © 2023.
- Magdy DM, Metwally A. Auto-titrating versus fixed-EPAP intelligent volume-assured pressure support (iVAPS) ventilation in patients with COPD and hypercapnic respiratory failure. Adv Respir Med 2021;89(3):277–83. doi:10.5603/ARM.a2021.0056
- Oscroft NS, Ali M, Gulati A, et al. A randomised crossover trial comparing volume assured and pressure preset noninvasive ventilation in stable hypercapnic COPD. COPD Dec 2010;7(6):398–403. doi:10.3109/15412555.2010.528084
- Shaughnessy GF, Gay PC, Olson EJ, Morgenthaler TI. Noninvasive volume-assured pressure support for chronic respiratory failure: a review. Curr Opin Pulm Med Nov 2019;25(6):570–7. doi:10.1097/mcp.0000000000000605
- Canpolat G, Ozgultekin A, Boran ÖF. Comparison of bilevel positive airway pressure and average volume-assured pressure support mode in terms of patient compliance and treatment success in hypercapnic patients. A cross-sectional study. Ann Ital Chir 2019;90:392–7.
- Canpolat G, Ozgultekin A, Turan G, et al. Does average volume-assured pressure support make any difference compared with BIPAP? Crit Care 2014;18(Suppl 1):P265. doi:10.1186/cc13455
- Crisafulli E, Manni G, Kidonias M, et al. Subjective sleep quality during average volume assured pressure support (AVAPS) ventilation in patients with hypercapnic COPD: a physiological pilot study. Lung Sep–Oct 2009;187(5):299–305. doi:10.1007/s00408-009-9167-1
- Ekkernkamp E, Storre JH, Windisch W, et al. Impact of intelligent volume-assured pressure support on sleep quality in stable hypercapnic chronic obstructive pulmonary disease patients: a randomized, crossover study. Respiration 2014;88(4):270–6. doi:10.1159/000364946
- Magdy DM, Metwally A. Effect of average volume-assured pressure support treatment on health-related quality of life in COPD patients with chronic hypercapnic respiratory failure: a randomized trial. Respir Res Mar 6 2020;21(1):64. doi:10.1186/s12931-020-1320-7
- Nilius G, Katamadze N, Domanski U, et al. Non-invasive ventilation with intelligent volume-assured pressure support versus pressure-controlled ventilation: effects on the respiratory event rate and sleep quality in COPD with chronic hypercapnia. Int J Chron Obstruct Pulmon Dis 2017;12:1039–45. doi:10.2147/copd.S126970
- Oscroft NS, Chadwick R, Davies MG, et al. Volume assured versus pressure preset non-invasive ventilation for compensated ventilatory failure in COPD. Respir Med Oct 2014;108(10):1508–15. doi:10.1016/j.rmed.2014.07.010
- Review Manager (RevMan). Version Web Version. 2024. Available at revman.cochrane.org
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials Feb 1996;17(1):1–12. doi:10.1016/0197-2456(95)00134-4
- Chronic obstructive pulmonary disease (COPD). Accessed September 17, 2024, 2024. https://bestpractice.bmj.com/topics/en-us/7/criteria
Article citation: Alam A, Nugent K. Average volume-assured pressure support versus fixed pressure support in chronic hypercapnic respiratory failure: A systematic review and meta-analysis. The Southwest Respiratory and Critical Care Chronicles 2024;12(53):19–26
From: Department of Internal Medicine, Texas Tech University Health Sciences Center, Lubbock, Texas
Submitted: 8/15/2024
Accepted: 9/25/2024
Conflicts of interest: none
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.