INTRODUCTION
The management of patients with congestive heart failure (CHF) initially requires an evaluation to establish the diagnosis, and the Framingham Heart Failure criteria can be used to identify patients who have a high likelihood of having heart failure. The next step involves echocardiography to determine cardiac structure and function, particularly left ventricular ejection fraction. The clinical status of a patient with heart failure can then be determined with the New York Heart Association functional classification or the Kansas City Heart Failure classification. Medical management involves treatment of increased afterload, inotropic support, when possible, reversal of ischemia, when present, and increased preload in some cases. Measuring the volume status and extravascular edema provides essential information for therapeutic decisions. Ultrasound offers a rapid and relatively simple noninvasive method to determine volume status and the presence or absence of extravascular fluid in the lungs and subcutaneous tissue. This review will analyze the use of ultrasound to identify and quantify pulmonary and subcutaneous edema in patients with CHF.
LIMITATIONS OF ECHOCARDIOGRAMS IN ASSESSING VOLUME AND EDEMA
Patients with CHF usually have abnormal echocardiograms, which provide valuable information about cardiac structure and function. Echocardiography serves as the gold standard for evaluating left ventricular systolic dysfunction and recognizing systolic heart failure and provides critical data on cardiac parameters, including ejection fraction, ventricular dimensions, and valvular function.1 However, these studies have significant limitations in assessing a patient’s volume status and the specific location of edema, which is crucial for the effective management of CHF patients. While echocardiography may indicate elevated filling pressures, these pressures do not always correlate with intravascular volume expansion, and studies have shown that in patients with elevated filling pressures, approximately one-third may have normal total blood volumes.2
Furthermore, echocardiography has several technical and clinical limitations. Patient-specific factors can affect image quality, leading to suboptimal acoustic windows and potentially inaccurate measurements. There is also a high likelihood of producing variable volume and ejection fraction results in repeated testing, especially with 2D imaging. Ejection fraction measurements are susceptible to inaccurate tracing and load dependence, offering little value in heart failure with preserved ejection fraction (HFpEF) for both diagnosis and prognosis. Doppler-based techniques for assessing diastolic function are heavily dependent on the angle of insonation and can be affected by factors like arrhythmias, mitral insufficiency, and loading conditions.1
The inability of echocardiography to directly assess volume status or to provide specific information about the location of edema in CHF patients is a significant clinical limitation.2 Edema can develop in several locations, including the lungs (pulmonary edema) and subcutaneous tissue in the lower extremities, depending on the nature and progression of heart failure.1 The lack of precise edema localization can hinder targeted treatment approaches. These limitations highlight the need for complementary diagnostic tools to provide a more comprehensive assessment of CHF patients, particularly to determine volume status and edema localization. Such information is crucial for guiding appropriate therapeutic interventions and improving patient outcomes in managing CHF.
Understanding volume status and the location of edema in CHF patients is vital for several reasons. Accurate assessment of volume status is critical for guiding treatment strategies, particularly with diuretic therapy and fluid management. Volume overload, or hypervolemia, is a frequent condition among CHF patients, especially those with increasing age, nutritional deficiencies, renal disease, or poor medication adherence. The location of edema provides important clinical information; rapid development of pulmonary congestion can indicate acute decompensation requiring immediate intervention, while generalized edema that develops gradually often signifies a more chronic process.2
In addition, fluid overload in CHF patients is closely associated with prognosis, reflecting the severity of disease progression, the exhaustion of neurohormonal compensatory mechanisms, and the potential for organ dysfunction due to the mechanical effects of edema. Monitoring volume status and edema helps assess the effectiveness of ongoing treatments, with the resolution of edema indicating successful management and persistent or worsening edema suggesting the need for treatment adjustment. Understanding the pattern and location of edema can also aid in differentiating CHF from other conditions that cause fluid retention, ensuring appropriate diagnosis and treatment.2 Thus, accurate assessment of volume status and edema location in CHF patients is essential for tailoring treatment strategies, evaluating disease progression, and ultimately improving patient outcomes in managing this complex cardiovascular condition.
ASSESSING VOLUME STATUS BASED ON INFERIOR VENA CAVA DIMENSIONS
Ultrasound of the inferior vena cava (IVC) has become an essential non-invasive tool for evaluating volume status in patients with medical disorders, including CHF. This technique provides valuable insights into intravascular volume and supports clinical decision-making and patient management. Key parameters measured in IVC ultrasound include the maximum IVC diameter (IVCmax) and the IVC collapsibility index (IVC CI) Figure 1.3 Interpretation of these measurements is critical for assessing fluid status. An IVCmax < 2.1 cm that collapses more than 50% during respiration usually indicates the absence of volume overload. Conversely, an IVC CI < 20% with a moderate to large IVC diameter suggests that intravascular volume depletion is unlikely. These criteria apply to both spontaneously breathing patients and patients on mechanical ventilation.3
Inferior vena cava ultrasound has shown particular promise in managing acute decompensated heart failure (ADHF). One study involving 80 ADHF patients demonstrated a significant increase in IVC CI from 19% to 25% (P = .001) within three hours of treatment, accompanied by a decrease in B-lines across all lung zones (B-lines are discussed in subsequent sections) (P = 0.001). Patients requiring hospitalization had lower IVC CI and more B-lines compared to those who were discharged. Notably, IVC ultrasound outperformed traditional methods, such as B-type natriuretic peptide levels, ejection fraction, and chest X-rays in diagnosing and evaluating the severity of ADHF.4 Other studies have highlighted the utility of IVC ultrasound in different heart failure subtypes. A multicenter study of 314 ADHF patients found that those with reduced ejection fraction (HFrEF) had a higher initial volume overload, as evidenced by lower IVC CI and increased B-lines, compared to patients with preserved ejection fraction (HFpEF). Following diuretic therapy, HFrEF patients showed a more significant increase in IVC CI and a reduction in B-lines.5
The prognostic value of IVC ultrasound is supported by a systematic review of 24 studies involving 1,900 hospitalized ADHF patients. This review revealed that a smaller IVC CI was associated with a 2.5-fold higher risk of readmission and mortality, while a greater number of B-lines was linked to a 1.5-fold increased risk of these outcomes.6 Similarly, the PROFUND-IC Registry Analysis of 389 elderly ADHF patients demonstrated that an IVC CI < 50% was associated with a 2.3-fold increase in hospital admissions over the prior year, a 3.1-fold increase in in-hospital mortality, and a 2.8-fold higher 30-day mortality rate (P < 0.04). Conversely, an IVC CI > 50% emerged as the strongest predictor of 30-day survival (odds ratio for mortality 0.359, P = 0.034).7
Despite its advantages, IVC ultrasound has limitations that must be considered. One common challenge is the potential difficulty in visualizing the IVC, which can lead to inaccurate measurements. For instance, in cases of severe volume depletion, the IVC may collapse completely, leading to misidentification of the aorta as the IVC. Accurate identification requires visualizing both vessels together.3 Moreover, various clinical factors can influence the interpretation of IVC findings. If a patient presents with conditions that typically cause IVC distention, but the IVC is small or collapsible, intravascular hypervolemia is unlikely. Conversely, a distended IVC in a context in which collapse is expected may indicate hypervolemia. Thus, integrating IVC ultrasound findings with the overall clinical picture is essential to avoid misinterpretation.3
IDENTIFYING EDEMA SITES WITH LUNG AND SUBCUTANEOUS TISSUE ULTRASOUND
PULMONARY EDEMA
Lung ultrasound can provide important information about the accumulation of fluid in the lung parenchyma and the pleural space.1 This procedure can be done in the office, in the emergency department, and in the hospital. This provides a rapid assessment of possible explanations for respiratory symptoms, such as dyspnea. Studies have used different numbers of lung zones for analysis. The best approach for clinical work likely involves the analysis of 8 zones located in anterior, midline, and posterior lung fields in the right and left lungs. The pleural line provides the initial site for analysis of the lung parenchyma. If this line seems intact and is sliding during respiration, then the plural surface is normal and a pneumothorax is unlikely. If the lung is normal and well aerated, there may be equally spaced A-lines below the pleural line that represent reverberations of the pleural line. These do not represent any ongoing active disease or lung injury, and the clinician can conclude that pulmonary edema is unlikely.
Patients with pulmonary edema have B-lines, which are vertical hyperechoic lines that radiate from the pleura through the depth of the lung in that particular field Figure 2. Patients with pulmonary edema usually have more than 3 B-lines in more than 2 zones in both lungs. The total number of B-lines reflects the degree of fluid accumulation. In some cases, there are so many B-lines that it is difficult to count them, and the clinician should try to estimate the percent of the lung parenchyma involved. However, these abnormalities are not specific to pulmonary edema and can occur in ARDS, interstitial pneumonitis, and interstitial fibrosis, as well as in focal areas of pneumonia. The clinician should also review the ultrasound to determine if there are any areas of consolidation with air bronchograms that would suggest pneumonia.
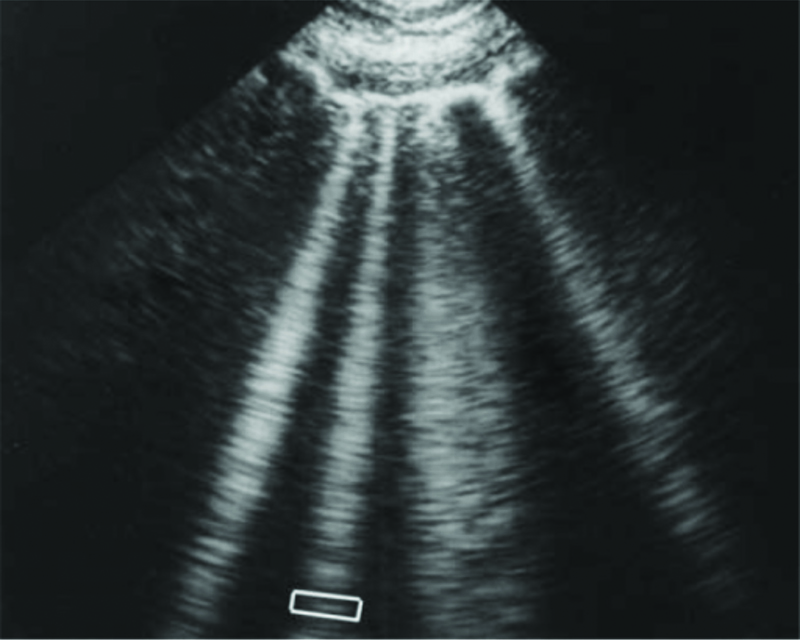
Figure 2. Ultrasound of lung B-lines.
Lung rockets. This sign is highly relevant in the lung ultrasound in critically ill patients. It shows here four or five B-lines. The B-line is a comet-tail artifact, arising from the pleural line, hyperechoic like the pleural line, spreading out without fading to the edge of the screen, well-defined, erasing the A-lines, and moving in concert with lung sliding. Three or more B-lines are called lung rockets, and are equivalent to interstitial syndrome. They are used to differentiate the different types of acute respiratory failure, and as help in managing acute circulatory failure.
Lichtenstein DA. Lung Ultrasound in the Critically Ill Neonate. Curr Pediatr Rev. 2012 Aug;8(3):217–223. Downloaded from OPENi image collection at Texas Tech University Health Sciences Center library on January 5, 2025.
Analysis of the pleural space provides additional information about intrathoracic fluid accumulation. Bilateral pleural effusions support the diagnosis of congestive. Effusions that have a simple anechoic character are consistent with a transudate. Focal unilateral effusions, especially with septations, suggest an exudative process, possibly explained by a parapneumonic effusion or metastatic disease to the pleura.
SUBCUTANEOUS EDEMA
Zhang et al. used a standardized protocol to determine subcutaneous edema in patients admitted to a medical intensive care unit in China.8 This study included 145 critically ill patients who were evaluated with ultrasound and a standardized “pitting” test to determine the presence of edema. Forty patients (27.6%) in this cohort had subcutaneous edema. Ultrasound identified subcutaneous edema more frequently than the pitting test (36.3% versus 30.8%; p = 0.002). The ultrasound was also used to determine the severity of the edema and distribution in specific regions of the body, including the abdominal wall, thighs, chest wall, calves, and hands. Thirty-nine patients (97.5%) had abdominal wall edema, which was most notable in the gravity-dependent zones of the abdominal wall. Twenty-nine patients had edema in their hands, and 28 patients had edema in their thighs. The median scores for the degree of edema were highest in the hands, abdominal wall, and thighs; the hands had the highest grade of edema. A diagnosis of sepsis, an increased APACHE 2 score (>15), an elevated NT-proBNP (>450 pg/ml), and an elevated creatinine (>1.47 mg/dL) were independent risk factors for the development of subcutaneous edema in these patients. The ultrasound edema score and the need for mechanical ventilation predicted mortality in an adjusted analysis. This study was not limited to patients with heart failure, and the pathogenesis of edema formation in these patients likely involved reduced oncotic pressures, increased capillary permeability, increased capillary hydrostatic pressures, and lymphatic obstruction. The risk factors for developing subcutaneous edema in these patients support the idea that pathogenesis is multifactorial. A subcutaneous edema score provides a global index of systemic disorders and should lead the clinician to review relevant factors and possibly change treatment strategies. However, edema formation in an acutely ill patient likely reflects a non-steady state condition and dynamic serial assessment is important.
THE ROLE OF POINT-OF-CARE ULTRASOUND IN HEART FAILURE MANAGEMENT
Point-of-care ultrasound in patients with congestive heart failure can provide essential information for the diagnosis of this syndrome, the management of patients with heart failure, and predictions of outcomes such as mortality and readmission to the hospital.
DIAGNOSIS
Point of care ultrasound (POCUS) demonstrated high diagnostic accuracy for heart failure with the sensitivity and specificity reaching 90–100% when combining IVC measurement with lung ultrasound assessment of B-lines or pleural effusion.9 This suggests POCUS could be a valuable tool for rapid diagnosis in emergency settings.
RESPONSE TO TREATMENT
Studies found that IVC diameter changed significantly following diuretic administration, with measurable differences observed as early as 1–2 hours post-treatment.9 This indicates that POCUS may be useful for monitoring volume status and treatment results in heart failure patients.
PREDICTION
Several studies showed that POCUS parameters, particularly IVC measurements, were predictive of outcomes such as readmission and mortality.9–11 For example:
- An IVC maximum diameter >2.0 cm at discharge was associated with higher odds of readmission;10
- The presence of ≥15 B-lines on lung ultrasound was associated with a 2.5 times higher likelihood of readmission within 3 months; and11
- Patients with ≥12 B-lines before discharge had a significantly higher risk of events at 6 months.10
Yampolsky et al. analyzed 15 studies that tried to determine the utility of ultrasound in identifying patients with heart failure, the use of ultrasound to determine responses to treatment, and the use of ultrasound to predict outcomes.11 These authors found that ultrasound had a diagnostic sensitivity and specificity of 90 to 100% when IVC measurements were coupled with lung ultrasound, assessing the presence of B-lines or pleural effusions. They found that measuring IVC diameter helped determine diuretic responses during treatment. Finally, they reported that six studies determined that ultrasound could predict long-term mortality and or hospital readmissions. For example, Miller found that an IVC collapsibility index of less than 25% had a sensitivity of 69%, a specificity of 89%, a positive likelihood ratio of 6.2, and a negative likelihood ratio of 0.35 in the prediction of heart failure.12 One study reported that visual estimation of left atrial volume, left ventricular volume, and left ventricular ejection fraction also provided useful information.13 Consequently, these studies indicate that clinicians need to develop the skills necessary to identify B-lines, pleural effusions, and IVC diameter to help diagnose and manage heart failure.
Panisello-Tafalla et al. published a systematic review of the use of lung ultrasound in the management of patients with heart failure.10 This study included 33 articles; 19 articles analyzed the use of lung ultrasound in prognostic assessment, 11 articles focused on diagnostic assessment, and 2 studied the use of ultrasound in therapeutic guidance. This review demonstrated that lung ultrasound could detect subclinical pulmonary edema, which has prognostic significance in admission to the hospital and mortality. In addition, lung ultrasound had a higher diagnostic accuracy than chest x-ray combined with NT pro-BNP. They concluded that ultrasound could provide early detection of decompensated heart failure both at discharge from the hospital and in outpatient evaluation. In addition, a lung ultrasound was more accurate than a clinical assessment in making a diagnosis of pulmonary congestion.
These findings suggest that POCUS could help identify high-risk patients requiring closer follow-up. However, there is some variability in the specific cutoff values and parameters used across studies. Overall, the evidence supports the potential utility of POCUS as a diagnostic, monitoring, and prognostic tool in heart failure management, though further standardization of techniques and interpretation may be needed.
In most cases, ultrasound images do not provide exact and specific diagnoses. Consequently, their use in the management of patients depends on information developed from clinical studies, and clinicians should analyze the characteristics of a study before accepting the information and ask questions about the study design. Is the study focused on the diagnosis of CHF, the response to treatment, and the prediction of adverse outcomes? These ultrasound images do not provide definitive diagnostic results and must be interpreted in the context of both the patient’s presentation and the supporting information usually from laboratory tests. In addition, clinical studies need to use well-defined patient cohorts. Do the patients have acute heart failure or chronic heart failure? Is information collected, e.g., IVC dimensions, analyzed as a continuous variable or as a categorical variable? If a categorical definition is used to identify an abnormal result, what is the cut point for that definition? Studies using ultrasound to support or confirm the diagnosis of CHF must have a solid gold standard for comparison. Studies using ultrasound to determine therapeutic responses must have a predefined and easily determined definition of the response in question. Studies using ultrasound to predict outcomes can use readmission to the hospital, adjustments in the therapeutic regimens, or death as outcomes. Finally, the location of the patient at the time of study entry is important. Studies on hospitalized patients are probably easier to develop and complete. Statistical analysis will also have to adjust for major clinical disorders associated with outcomes in cardiac patients, including age, echocardiogram results, atrial fibrillation, chronic kidney disease, and diabetes.
DISCUSSION
The initial evaluation of a patient presenting with dyspnea who possibly has CHF should include a detailed history of his or her current symptoms and past history to collect information that would support the diagnosis of an underlying cardiac disorder. The next step usually involves a careful physical examination and probably a chest x-ray. Additional testing will depend on whether or not the current presentation represents an acute disorder or a chronic disorder.
Information about the volume status of their patients helps clinicians confirm their working diagnoses and develop and follow treatment plans. In particular, the identification of B-lines in the lungs helps identify patients with pulmonary edema. The most obvious situations involve patients with B-lines and a dilated inferior vena cava without collapsibility, a patient with no B-lines and a normal inferior vena cava, and a patient with no B-lines and a reduced inferior vena cava size with increased collapsibility. These possibilities and their interpretation are outlined in the Table 1. However, this analysis and classification becomes much more difficult in patients with complicated comorbidity in addition to a cardiac disorder. For example, patients with anemia likely have decreased volume status. Patients with sepsis have increased capillary permeability and will have increased subcutaneous tissue edema and possibly non-cardiac pulmonary edema. In addition, the information collected by ultrasound may change during the hospital course based on either treatment and resolution or the development of complications, such as a kidney injury with overload or sepsis with capillary permeability and increased interstitial fluid. The clinician will likely need to make several measurements and correlate them with clinical status at the bedside.
Table 1. Ultrasound Categories in Heart Failure
| Ultrasound Results |
Clinical Condition* |
| Normal IVC, no peripheral edema |
Normal volume status, no extravascular fluid |
| Increased IVC, no peripheral edema |
Early phase of heart failure or renal failure |
| Increased IVC, edema |
Advanced heart failure or renal failure |
| Decreased IVC, no edema |
Hypovolemia |
| Decreased IVC. peripheral edema |
Treated heart failure or renal failure |
The use of ultrasound to identify extravascular fluid in either the lung or subcutaneous tissue provides important information about a patient’s clinical status. The measurement of the volume or dimensions of the inferior vena cava also provides information about the volume status of the patient. The use of this testing requires contact with the patient and provides an opportunity to review symptoms and physical examination. This should improve diagnostic assessment and the measurement of response to treatment.
REFERENCES
- Gargani L, Girerd N, Platz E, et al. Lung ultrasound in acute and chronic heart failure: a clinical consensus statement of the European Association of Cardiovascular Imaging (EACVI). Eur Heart J Cardiovasc Imaging 2023 Nov 23;24(12):1569–1582. doi: 10.1093/ehjci/jead169.
- Pellicori P, Platz E, Dauw J, et al. Ultrasound imaging of congestion in heart failure: examinations beyond the heart. Eur J Heart Fail 2021 May;23(5):703–712. doi: 10.1002/ejhf.2032.
- Kaptein EM, Kaptein MJ. Inferior vena cava ultrasound and other techniques for assessment of intravascular and extravascular volume: an update. Clin Kidney J 2023 Jun 29;16(11):1861–1877. doi: 10.1093/ckj/sfad156.
- Hacıalioğulları F, Yılmaz F, Yılmaz A, et al. Role of point-of-care lung and inferior vena cava ultrasound in clinical decisions for patients presenting to the emergency department with symptoms of acute decompensated heart failure. J Ultrasound Med 2021 Apr;40(4):751–761. doi: 10.1002/jum.15447.
- Cogliati C, Ceriani E, Gambassi G, et al. Phenotyping congestion in patients with acutely decompensated heart failure with preserved and reduced ejection fraction: The Decongestion duRing therapY for acute decompensated heart failure in HFpEF vs HFrEF- DRY-OFF study. Eur J Intern Med 2022 Mar;97:69–77. doi: 10.1016/j.ejim.2021.11.010.
- Arvig MD, Laursen CB, Jacobsen N, et al. Monitoring patients with acute dyspnea with serial point-of-care ultrasound of the inferior vena cava (IVC) and the lungs (LUS): a systematic review. J Ultrasound 2022 Sep;25(3):547–561. doi: 10.1007/s40477-021-00622-7.
- Pérez-Herrero S, Lorenzo-Villalba N, Urbano E, et al. Prognostic significance of lung and cava vein ultrasound in elderly patients admitted for acute heart failure: PROFUND-IC Registry Analysis. J Clin Med 2022 Aug 5;11(15):4591. doi: 10.3390/jcm11154591.
- Zhang W, Gu Y, Zhao Y, et al. Focused liquid ultrasonography in dropsy protocol for quantitative assessment of subcutaneous edema. Crit Care 2023 Mar 18;27(1):114. doi: 10.1186/s13054-023-04403-y.
- Russell FM, Rutz M, Pang PS. Focused Ultrasound in the Emergency Department for Patients with Acute Heart Failure. Card Fail Rev 2015 Oct;1(2):83–86. doi: 10.15420/cfr.2015.1.2.83.
- Panisello-Tafalla A, Haro-Montoya M, Caballol-Angelats R, et al. Prognostic significance of lung ultrasound for heart failure patient management in primary care: a systematic review. J Clin Med 2024 Apr 23;13(9):2460. doi: 10.3390/jcm13092460.
- Yampolsky S, Kwan A, Cheng S, et al. Point of Care Ultrasound for diagnosis and management in heart failure: a targeted literature review. POCUS J. 2024 Apr 22;9(1):117–130. doi: 10.24908/pocus.v9i1.16795.
- Miller WL. Fluid volume overload and congestion in heart failure: time to reconsider pathophysiology and how volume is assessed. Circ Heart Fail. 2016 Aug;9(8):e002922. doi: 10.1161/CIRCHEARTFAILURE.115.002922.
- Carlino MV, Paladino F, Sforza A, et al. Assessment of left atrial size in addition to focused cardiopulmonary ultrasound improves diagnostic accuracy of acute heart failure in the Emergency Department. Echocardiography. 2018 Jun;35(6):785–791. doi: 10.1111/echo.13851.
Article citation: Tran V, Swarna S, Nugent K. The use of ultrasound to determine volume status and identify sites of edema in patients with heart failure. The Southwest Respiratory and Critical Care Chronicles 2025;13(54): 1–8
From: Department of Internal Medicine, Texas Tech University Health Sciences Center, Lubbock, Texas
Submitted: 11/24/2024
Accepted: 1/6/2025
Conflicts of interest: none
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.